Abstract
Aims: Drug-eluting stents (DES) reduce late lumen loss compared to bare metal stents but were not able to eradicate in-stent restenosis (ISR) fully. Vascular endothelial growth factor (VEGF) may inhibit late lumen loss through accelerated reendothelialisation, but may also promote neointima formation by proinflammatory effects. The aim of this study was to evaluate whether endogenous plasma levels of VEGF are associated with development of ISR after implantation of DES.
Methods and results: We studied 85 patients who were treated with 159 DES. VEGF plasma levels were determined before and 24 hours after PCI. During the eight-month follow-up period, two patients (2.4%) died of cardiovascular causes and 12 patients (14.5% of patients, 7.6% of stents) developed angiographic ISR. Basal VEGF plasma levels were not different in patients with and without ISR at follow-up. In contrast to patients without ISR, VEGF increased significantly upon PCI in patients with ISR (p<0.005). Patients with a decrease of VEGF after PCI had a restenosis rate of 2.4% compared to a restenosis rate of 26.2% in patients with an increase of VEGF after the procedure (p<0.05). This was independent from clinical and angiographic risk factors.
Conclusions: Basal plasma levels of VEGF are not associated with the development of ISR. However, an increase of VEGF after PCI is associated with a dramatically increased ISR rate after implantation of DES.
Introduction
Stenosis of the coronary arteries is a daily challenge for interventional cardiologists. The latest milestone in interventional cardiology was the introduction of drug-eluting stents (DES). However, DES were not able to eradicate in-stent restenosis (ISR) fully. Data from a recent study suggest an 18% restenosis rate in “real-world patients” even with drug-eluting stents1. DES reduce the rate of ISR compared to bare metal stents (BMS) through inhibition of migration and proliferation of coronary smooth muscle cells2,3. However, recent studies suggest that sirolimus and paclitaxel also inhibit endothelial cell proliferation4,5, leading to delayed healing of the endothelium with inhibition of reendothelialisation6,7, which may have the disastrous consequence of late stent thrombosis and which could also promote the development of ISR7.
As the main functions of vascular endothelial growth factor (VEGF) are to promote survival, proliferation and migration of endothelial cells8-10, VEGF appears to be critically involved in the modulation of vascular healing and thereby may prevent ISR. On the other hand, VEGF may also promote the development of in-stent restenosis through proinflammatory effects11-13 and an increase of adventitial neovascularisation14,15. In addition, VEGF may recruit endothelial progenitor cells16,17 that may improve reendothelialisation but may participate in neointima formation. Therefore, it is not surprising that modulation of VEGF in animal models showed divergent effects on neointima formation after injury18-24. In contrast, VEGF-eluting stents have shown mostly promising results25-30.
Sirolimus has been shown to decrease VEGF in vitro31 and in the coronary circulation32. A recent study showed that VEGF mRNA expression in circulating monocytes was decreased one month after implantation of sirolimus-eluting stents as compared to bare metal stents. In addition, VEGF mRNA levels one month after intervention were associated with late lumen loss in bare metal as well as in sirolimus-eluting stents33. In contrast, it has been reported that paclitaxel may increase VEGF in vitro through reactive oxygen species production34.
Taking the previous experimental and clinical data together, the hypothesis of this study is that circulating, luminal VEGF, measured before the intervention, may be associated with increased reendothelialisation after stent implantation and may thereby be beneficial in the prevention of restenosis. In addition, we propose that the increase of VEGF 24 hours after PCI reflects, at least in part, the VEGF content within the target lesion and may thereby be associated with increased neointima formation by proinflammatory mechanisms and increased adventitial neovascularisation.
Methods
PATIENTS
Blood samples were taken prospectively from all patients with stable coronary artery disease who underwent elective PCI (n=147). The type, number, length and size of the stent(s) implanted were left to the discretion of the interventionalist. First-generation DES were used and patients received either paclitaxel-eluting stents (TAXUS® Express™; Boston Scientific Corp., Natick, MA, USA) or sirolimus-eluting stents (CYPHER™; Cordis Corp., Miami Lakes, FL, USA), respectively. All patients with DES only were asked to participate in this study and we included all 85 patients who gave their informed consent for follow-up angiography35. The study was approved by the institutional ethics committee.
Patients with acute coronary syndrome within three months before angioplasty were excluded. Exclusion criteria also comprised concurrent severe illness (such as cancer, hepatic or renal disease or chronic infections), PCI for restenosis and unsuccessful procedure (i.e., >50% diameter stenosis after the intervention). PCI was performed according to standard techniques by experienced interventionalists only. Aspirin and unfractionated heparin were administered as per standard practice. Clopidogrel therapy was either started on the day before angiography or immediately after stent implantation with four tablets (300 mg). After the procedure, patients were maintained on aspirin 100 mg indefinitely, and clopidogrel 75 mg for at least six months. Other medications such as ß-blockers, statins, and angiotensin-converting enzyme inhibitors were given as appropriate.
ENDPOINTS AND DEFINITIONS
Angiographic restenosis was evaluated at six to eight months of follow-up. The off-line quantitative coronary angiographic analysis was performed with an automated edge-detection system (QCA-CMS V 6.0; Medis Medical Imaging Systems, Leiden, The Netherlands) by a single blinded expert. The contrast-filled, non-tapered catheter tip was used for calibration. The reference diameter was measured by interpolation. Minimal lumen diameter was measured within the stent and within the 5 mm proximal and distal edges of the stent. The endpoint of the study was binary angiographic restenosis (diameter stenosis of at least 50% based on in-segment analysis) at follow-up angiography.
LABORATORY MEASUREMENT
After informed consent was obtained, two blood samples were taken under fasting conditions at baseline (one day before PCI) and 24 hours after PCI. Therefore, venous blood was drawn from the antecubital vein with minimal tourniquet pressure into EDTA tubes. After centrifugation (4°C; 3,000xg for 15 min) plasma samples were stored at –70°C until use.
VEGF-165 antigen was determined by ELISA (R&D Systems, Minneapolis, MN, USA). The limit of detection was 9 pg/mL and the intra- and interassay variation was <10%. We used high-sensitivity assays for measurement of serum C-reactive protein (CRP) (DADE Behring, Vienna, Austria) with a lower detection level of 0.03 mg/dL and a coefficient of variation of 4.6%. Tumour necrosis factor alpha (TNF-α) was measured by ELISA (R&D Systems). The limit of detection was 0.106 pg/mL and the intra- and interassay variation was <10.4%. Monocyte chemotactic protein-1 (MCP-1) was determined by ELISA (Bender MedSystems, Vienna, Austria). The limit of detection was 2.3 pg/mL and the intra- and interassay variation was <8%.
STATISTICAL ANALYSES
The hypothesis of this study was that either baseline VEGF plasma levels or the increase of VEGF levels upon PCI show a difference of at least 50% in patients with and without ISR. A preliminary cohort of 20 patients showed mean VEGF levels of 780.8±375.5 pg/mL. Calculation of sample size revealed that, with an expected “real-world” restenosis rate of 10%, 80 patients were needed to detect a 50% difference in VEGF levels before PCI between patients with and without ISR with a power of 80% and a significance level (two-tailed) of 0.0536. In patients with multilesion interventions, only the lesion with the highest late lumen loss was included in the analysis. Categorical variables are summarised as counts or percentages and were compared by the χ2 or Fisher’s exact test. Continuous variables are expressed as median (interquartile range) or as mean±standard deviation. Non-parametric data (assessed by the Kolmogorov-Smirnov test) were compared by the Mann-Whitney test and parametric data were compared by the unpaired Student’s t-test. Changes before and after PCI were determined by the Wilcoxon signed-rank test for paired samples. Pearson’s correlation was used to correlate VEGF levels with late lumen loss after log10 transformation. Since a multivariable analysis was not feasible with only 12 ISR events, we adjusted only for the variable that was identified as the strongest confounding variable. As strongest confounder we selected the variable which implied the strongest modification of the odds ratio of VEGF towards one among all bivariable models. Receiver operating characteristic curves were calculated for in-stent restenosis using angiographic and procedural or clinical characteristics. Variables were selected if they either: a) had a clinically plausible relation with the outcome, or b) appeared to be imbalanced between patients with and without restenosis indicated by a p-value <0.20. A p-value of <0.05 (two-tailed) was considered statistically significant. All statistical analyses were performed with the statistical software package SPSS 11.0 (SPSS Inc., Chicago, IL, USA).
Results
PATIENT CHARACTERISTICS
One hundred and twenty-nine paclitaxel-eluting stents were implanted in 63 patients (75%) and 30 sirolimus-eluting stents were used in 22 patients (25%). During follow-up, two patients (2.4%) died of cardiovascular causes without evidence of stent thrombosis, and ISR occurred in 12 patients (14.5% of patients, 7.6% of stents). The mean age of the patients was 64 years, and 79.5% were male. Patients with and without restenosis showed no significant differences in their baseline as well as in their angiographic characteristics (Table 1, Table 2).
PLASMA LEVELS OF VEGF IN PATIENTS WITH AND WITHOUT RESTENOSIS
Plasma levels of VEGF before the procedure (Figure 1) were not different in patients with and without ISR at follow-up angiography. Whereas PCI did not affect VEGF plasma levels in patients without ISR (before PCI: 584.4 [314.4-844.9], 24 hours after PCI 515.1 [302.5-770.5] pg/mL), VEGF plasma levels increased significantly in patients with ISR from 344.4 (170.2-841.9) pg/mL to 809.1 (618.3-1,066.2) pg/mL (p<0.005). Accordingly, 24 hours after PCI, patients with ISR at follow-up angiography showed significantly higher plasma levels of VEGF (Figure 1). Figure 2 shows follow-up angiographies, intravascular ultrasound images and corresponding VEGF plasma levels of a patient without and with ISR, respectively. Type of ISR (focal n=7, diffuse n=5) was not significantly associated with VEGF plasma levels before or after PCI (data not shown).
Figure 1. Plasma levels of VEGF before (red boxes) and 24 hours after PCI (grey boxes) in patients without and with angiographic in-stent restenosis at follow-up. Box plots indicate median, IQR (range from the 25th to the 75th percentile) and total range, circles indicate outliers. p<0.05 no restenosis vs. restenosis; p<0.005 before vs. 24 hours after PCI.
Figure 2. Follow-up angiography and intravascular ultrasound image at follow-up of a patient without (A, C) and with (B, D) in-stent restenosis. Corresponding VEGF plasma levels before and 24 hours after PCI of the patient without (E) and with ISR (F). DS: diameter stenosis; IH: intimal hyperplasia; MLD: minimal lumen diameter
RESTENOSIS RATES IN PATIENTS WITH INCREASE OF VEGF PLASMA LEVELS POST PCI
Patients with a decrease of VEGF after PCI had a restenosis rate of 2.4% (1 of 41) compared to a restenosis rate of 26.2% (11 of 42) in patients with an increase of VEGF after the procedure (p<0.05). Logistic regression analysis revealed that this association was independent from clinical, angiographic and procedural risk factors (Table 3). The C-statistic for the occurrence of in-stent restenosis was the highest with 0.81 (0.69-0.92) for VEGF increase as compared to 0.76 (0.65-0.89) for angiographic and procedural risk factors and 0.73 (0.55-0.91) for clinical risk factors (Figure 3).
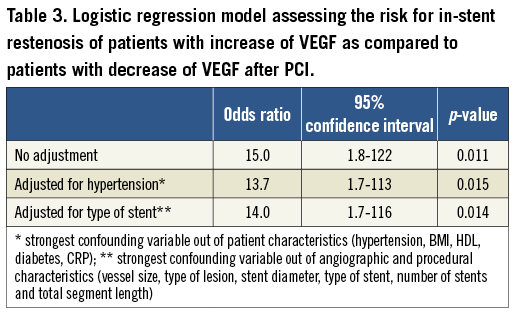
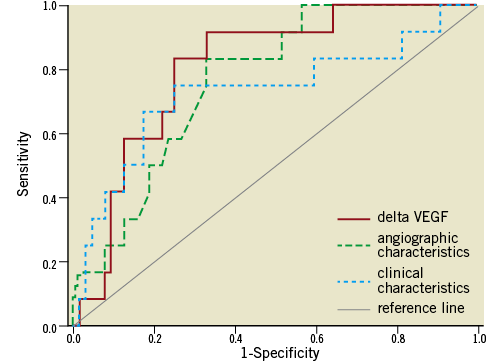
Figure 3. Receiver operating characteristics curves for the occurrence of in-stent restenosis for VEGF plasma levels (red solid line; increase pre vs. post), angiographic and procedural characteristics (green dashed line; vessel size, type of lesion, stent diameter, type of stent, number of stents and total segment length) and clinical characteristics (blue dotted line; hypertension, BMI, HDL, diabetes).
VEGF PLASMA LEVELS BEFORE AND AFTER PCI ARE NOT ASSOCIATED WITH INFLAMMATORY PARAMETERS
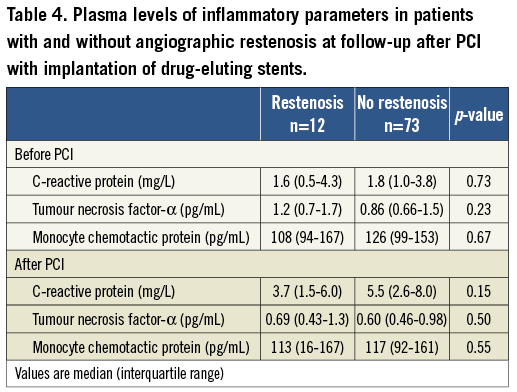
To test whether VEGF plasma levels before and after PCI are associated with inflammatory parameters we measured white blood cell count, CRP, TNF-α and MCP-1. Neither VEGF before PCI nor the increase of VEGF upon PCI were associated with the inflammatory markers or their increase (p=NS). Interestingly, white blood cell count, CRP, TNF-α and MCP-1 were not different in patients with and without ISR (Table 4).
Discussion
In this prospective “real-world” study with angiographic follow-up after implantation of DES we report that patients with ISR showed a significant increase in their VEGF plasma levels due to PCI. Patients with an increase of VEGF after PCI showed an increased risk for restenosis that was independent of clinical, angiographic and procedural risk factors.
In a recent study, VEGF mRNA expression of circulating monocytes one month after intervention was associated with late lumen loss in bare metal as well as in sirolimus-eluting stents33. We were able to demonstrate that VEGF plasma levels 24 hours after the intervention were already associated with development of ISR. It should be emphasised that patients in our study who were free of ISR at follow-up angiography showed a decrease of VEGF plasma levels upon PCI. In contrast, 91.7% of patients with ISR showed a significant increase of VEGF plasma levels caused by the procedure.
In our study, the C-statistic for VEGF was better as compared to the discriminatory ability of angiographic and procedural characteristics or clinical risk factors to predict in-stent restenosis. Measurement of VEGF as a biomarker before and 24 hours after PCI could be a new and easy tool for prediction of ISR. Patients who show an increase are at very high risk for the development of ISR. This could eventually trigger a closer monitoring of such patients including scheduling frequent clinical visits, stress tests or even repeat angiography.
Animal studies have suggested that the loss of arterial coverage by endothelium after injury may stimulate SMC proliferation and that increased reendothelialisation due to systemic VEGF overexpression may limit neointima formation18,21. We proposed that high basal, circulating VEGF may be associated with a reduced ISR rate. However, we could not detect a statistically significant difference in plasma levels of VEGF before PCI in patients with and without restenosis.
In a hyperlipidaemic rabbit model PCI increased VEGF expression in the arterial wall37, whereas stent implantation did not increase circulating VEGF levels in patients with stable coronary artery disease38. We want to speculate that the change of circulating VEGF reflects the expression of VEGF within the treated atherosclerotic plaque.
VEGF plasma levels did not correlate with circulating plasma levels of the inflammatory cytokine TNF-α, the chemokine MCP-1 or the acute phase protein CRP. In addition, all three inflammatory markers were not associated with the occurrence of ISR after implantation of DES. This is in line with previous studies that showed a role for inflammatory markers in prediction of ISR after implantation of BMS but failed to show an association of circulating inflammatory markers and ISR after treatment with DES.
The increase of VEGF observed in the group of patients who developed ISR in our study may reflect a higher basal content of VEGF in the treated plaque which may be released by the intervention, an acute local process upon stent implantation or a higher degree of local injury. It should be noted that VEGF within the arterial wall after vascular injury may promote smooth muscle cell proliferation19,23,24 and may increase adventitial neovascularisation that has been associated with increased neointima formation14,15. Thus, one might speculate that the increase of circulating VEGF in patients with future ISR may be due to expression of significant levels of VEGF locally in the arterial wall which may be absent or lower in patients without ISR.
Limitations
Some limitations of the present study have to be acknowledged. As the primary endpoint of this study was angiographic restenosis, we included only patients who gave their informed consent for control angiography. However, we do not believe that selection bias played a major role since these patients did not differ in respect to baseline characteristics and outcome from the total cohort of patients that had PCI with implantation of DES. Further, our study is necessarily of an observational nature. Accordingly, our results may be explained by unmeasured confounding factors. Therefore, we tried to control for baseline imbalances by multivariate modelling. However, the possibility of residual or undetected confounding, though small, cannot be ruled out completely. In addition, there was a range of three months in the timing of follow-up angiography (six to eight months) and the healing process might not have been completed in some patients with early follow-up angiography. However, FU time did not correlate with late lumen loss (r=–0.11, p=0.32). In contrast, there was a trend that patients who experienced ISR underwent FU angiography earlier, possibly triggered by clinical symptoms. Finally, this trial was not powered to detect a difference in stent type. Therefore, only a pooled analysis was performed. In addition, first-generation DES were used. Therefore we are not able to draw any conclusions regarding any associations between VEGF plasma levels and second or third-generation DES.
Conclusions
Whereas basal plasma levels of VEGF were not associated with the development of ISR, we present evidence that, in patients who developed ISR, VEGF plasma levels increased significantly due to PCI. We speculate that an increase of VEGF immediately after PCI might reflect an increased VEGF content of the target lesion and/or an increased inflammatory reaction at the site of the implanted stent and therefore might impact on the development of ISR.
Acknowledgements
This work was supported by the Ludwig Boltzmann Foundation for Cardiovascular Research and by the Association for the Promotion of Research in Arteriosclerosis, Thrombosis and Vascular Biology.
Conflict of interest statement
The authors have no conflicts of interest to declare.

