Abstract
Aims: Drug-eluting stents (DES) have become the first choice to treat BMS restenosis (ISR), replacing brachytherapy and all other available percutaneous approaches. Although markedly reduced, DES ISR still occurs and has been frequently treated with another DES, despite the lack of robust data supporting the safety and efficacy of this approach. We sought to compare the long term clinical outcomes of patients with BMS and DES ISR treated with another DES deployment.
Methods and results: Between May 2002 and January 2008 a total of 158 patients with BMS restenosis and 58 patients with DES restenosis were treated with a DES and enrolled in this registry. Primary endpoint included the cumulative occurrence of major adverse cardiac events (MACE=cardiac death, myocardial infarction and target-vessel revascularisation) and stent thrombosis. Baseline clinical aspects did not significantly differ between the groups. There was a trend toward a higher incidence of DM in the DES cohort (36.1% vs. 32.9%, p=0.1). Mean time between first procedure and restenosis was significant longer in the DES population (178±61 days vs. 140±38 days, p=0.02). At the end of the follow-up period, 92.6% of the patients with BMS-ISR and 86.3% of those with DES-ISR were free of MACE (p<0.001). Patients with DES ISR had significant more recurrence of ISR but equivalent rates of cardiac death, MI and stent thrombosis.
Conclusions: Percutaneous treatment of BMS or DES ISR with the implant of a DES represents a simple and safe approach with sustained long term results. However, the relatively high rate of ISR recurrence among patients with prior DES ISR demand the developing of more effective strategies for that subset of individuals.
Introduction
Since their introduction, excessive neointimal growth and restenosis have been the major drawbacks of bare-metal stents (BMS). Drug-eluting stents (DES) were primarily conceived to reduce BMS excessive neointimal tissue formation that ultimately resulted in repeated revascularisation procedures.
With their efficacy and superiority over BMS demonstrated in a variety of complex clinical and angiographic scenarios, DES were rapidly incorporated into clinical practice1-3 and became the treatment of choice for cumbersome situations, including BMS restenosis4-6.
Although infrequent, DES restenosis still occurs and the best approach to this adverse event is yet to be defined. However, placement of another DES has emerged as an attractive alternative despite the lack of robust evidence demonstrating the long term efficacy and safety of this approach.
In this current analysis we present the very long term (up to six years) clinical outcomes of DES for the treatment of previous BMS and DES restenosis.
Methods
Since the first DES was commercially approved in our country (May 2002), all consecutive patients treated solely with those devices have been enrolled in the non-randomised, single-centre, DESIRE (Drug-Eluting Stents In the REal World) registry. Details about this registry as well as its overall results have been previously published elsewhere7. In brief, clinical inclusion criteria were “all comers” for routine or emergency percutaneous coronary intervention (PCI) with >18 years of age. Angiographic inclusion criteria were the presence of at least one documented stenosis ≥50% (by visual estimation) in a native coronary vessel or graft (arterial or venous) suitable for PCI with drug-eluting stent implantation. There were no protocol pre-specified limitations concerning the number of target lesions and/or target vessels that could to be treated with DES. The DESIRE registry was conceived to evaluate the very long term clinical outcomes of DES used for the treatment of a large cohort of complex unselected patients. The study’s primary objective was the occurrence of major adverse cardiac events (MACE=cardiac death, myocardial infarction and target-vessel revascularisation) at the in-hospital and long term clinical follow-up.
In the present study, we included patients with either BMS or DES restenosis who received a DES as the treatment of choice. ISR was defined as ≥50% angiographic stenosis within or 5 mm proximal and distal to the stent.
Since this study is based on clinical endpoints, patients receiving both drug-eluting and bare-metal stents in the same procedure were excluded from the final analysis. Additionally, patients were excluded if the target lesion was previously treated for ISR and/or if it was located in a venous or arterial graft.
The study was approved by the institutional ethics committee. Written informed consent was obtained from all patients prior to the procedure. The institution and the participants did not receive any kind of financial support to develop this research.
Stenting procedure
All interventions were performed according to the current standard guidelines, and the final procedure strategy was entirely left upon operators’ discretion. Two different DES were available: (a) Sirolimus-eluting stent (Cypher™, Cordis, Johnson & Johnson, Warren, NJ, USA) in diameters ranging from 2.25 to 3.5 mm and lengths from 8 to 33 mm, and; (b) Paclitaxel-eluting stents (Taxus™, Boston Scientific Corporation, Natick, MA, USA) in diameters ranging from 2.25 mm to 4.0 mm and lengths from 8 to 32 mm. It is important to stress that the type of stent to be deployed, as well as the strategy to pre- and/or post-dilate, was left to the operator’s discretion. Athero-ablation techniques, cutting-balloon and other similar devices were not used in these patients.
Dual antiplatelet therapy, including loading dose of aspirin (200 to 325 mg) and thienopyridines (ticlopidine 250 mg B.I.D. or clopidogrel 300 to 600 mg) was started at least 24 hours before elective procedures, otherwise a loading dose of 600 mg of clopidogrel was given immediately prior to the intervention. Post-procedural aspirin was continued indefinitely and thienopyridine was maintained for 12 months.
During procedure, intravenous heparin (70 to 100 IU per kg) was administered after sheath insertion to maintain an activated clotting time >250 seconds. Use of additional medications during the procedure, including glycoprotein IIbIIIa inhibitors, was left at operator’s discretion. A 12-lead electrocardiogram was obtained: before the procedure, immediately afterwards, as well as 24 hours later. Blood sample laboratory analysis included creatine kinase cardiac enzymes (CK and CK-MB) before procedure (<24 hours) and 12-18 hours after treatment.
Angiographic analysis
After intracoronary nitrate administration (100-200µg), serial coronary angiography was obtained at baseline and post-procedural. Off-line quantitative coronary angiography (QCA) analysis was performed using the semi-automatic edge contour-detection computer analysis system CMS-GFT™ version 5.1 (Medis, Leiden, The Netherlands). The minimum lumen diameter (MLD) and the mean reference diameter (RD), obtained from averaging 5 mm “non-diseased” segments proximal and distal to the target lesion location(s), were used to calculate the diameter stenosis (DS=[1–MLD/RD] x 100). Acute gain was the change in MLD from baseline to final post-stent implantation angiogram.
All cine-angiogram images were analysed at the Hospital do Coração Angiographic Core Laboratory (São Paulo, Brazil) by experienced senior operators blinded to procedural data.
Endpoints, definitions and clinical follow-up
The primary objective of our study was the comparison of major adverse cardiac events (MACE) and stent thrombosis at very long term clinical follow-up of patients with BMS or DES ISR treated with another DES.
MACE was defined as cardiac death, non-fatal myocardial infarction (MI), and target lesion revascularisation (TLR). All deaths were considered to be cardiac unless a non-cardiac origin could be clearly established by clinical and/or pathological study. The diagnosis of MI was based on either the development of new pathological Q waves in ≥2 contiguous electrocardiogram leads and/or elevation of CK-MB isoenzyme >3 times the upper normal limit post-procedure during index hospitalisation, or cardiac enzyme elevation >2 times the upper normal limit thereafter. TLR was only based on the presence of symptoms and/or signs of ischaemia.
Stent thrombosis was classified as definite, probable and possible according to definitions proposed by the Academic Research Consortium (ARC)8, and was stratified as acute (<24 hours), sub-acute (24 hours to 30 days), late (1 to 12 months) and very late (> 1 year)
Angiographic success was defined as attainment of <20% residual stenosis by QCA in the treated segment post DES treatment. Procedural success was defined as angiographic success plus absence of MACE during hospitalisation. During the enrolment period, detailed demographic, clinical, angiographic and procedural information, including complications, were gathered for each patient.
Clinical follow-ups, by office appointment or phone call, were scheduled at 1, 3, 6 and 12 months after stent implantation, and then annually up to six years of the baseline procedure on the basis of information entered on case report forms at the time of the office visit/telephone contact. At the time of the follow-up, data were collected pertaining to current clinical status, concomitant drug-therapy and interim occurrence of the pre-defined adverse events. All phone follow-up data was collected by the same person especially trained to this task and blinded to the procedure results. Individual patient data was coded to prevent the identification of study participants.
Routine angiographic follow-up was not part of the study protocol. Therefore, all re-interventions were clinically (ischaemia)-driven.
Statistical analysis
Data are presented as mean ± 1 standard deviation (SD) or frequencies. Categorical variables were compared with the Chi-square Test. When the assumptions were broken, Fisher Exact Test was used. For continuous variables comparison, t test was used.
Cumulative event-free survival for MACE was demonstrated by Kaplan-Meier curve and the differences between the two study groups were assessed with the log-rank test. A value of p<0.05 was considered significant. Statistical analysis was performed using SPSS version 11.0 (Chicago, IL, USA).
Results
Between May 2003 and January 2008, a total of 2,500 patients (3,333 lesions) were treated solely with DES in our institution and consented to take part in the DESIRE Registry. Among the overall population, 292 patients with ISR were initially identified. Notably, there were 76 patients with ISR who had also developed significant lesion progression in other territories and/or the angiographic characteristics of the ISR were deemed inappropriate to PCI, and were referred to CABG, being excluded from this registry. The remaining 216 patients were deemed suitable for percutaneous re-intervention, matched the inclusion/exclusion criteria, and therefore, were enrolled in the present analysis (158 following BMS implantation and 58 after DES implantation). The vast majority of patients in both cohorts were symptomatic at the time of the additional revascularisation procedure (111 patients–70.2% in the BMS ISR group vs. 39 patients–67.2% in the DES ISR cohort, p=0.4).
Patients with DES ISR were slightly younger (59.5±9.8 vs. 62.6±11.5, p=0.08) and had higher, non-significant, prevalence of diabetes mellitus (36.1% vs. 32.9%, p=0.1) and medically treated hyperlipidaemia (79.3% vs. 67.1%. p=0.08). Table 1 contains detailed clinical profile of the patients enrolled into this analysis.
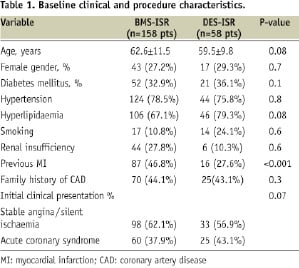
Table 2 shows the main procedure characteristics as well as pre- and post- intervention QCA data.
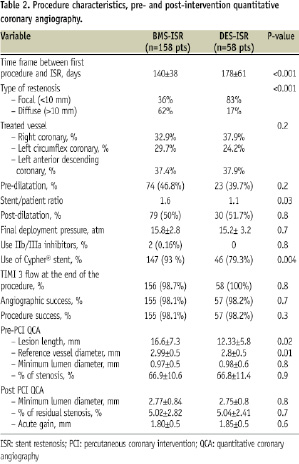
Diffuse restenosis pattern was more often observed among patients in the BMS ISR cohort (62% vs. 17%, p<0.001). Mean time between first procedure and restenosis was significant longer in the DES population (178±61 days vs. 140±38 days, p=0.02). Treatment of BMS ISR required more stents than the other cohort (stent/patient ratio of 1.6 vs. 1.1, p=0.01).
Overall, patients in the DES ISR arm had smaller target vessels (2.8±0.5 mm vs. 2.99±0.5 mm, p=0.02) and shorter lesions (12.33±5.8 mm [2.1–21.6 mm] vs. 16.6±7.3 mm[5.1–24.7 mm], p<0.001). At the end of the procedure, acute gain and residual stenosis were equivalent between the cohorts. Angiographic and procedure success was achieved in >98% of the cases in both groups (p=NS).
There was no difference regarding in hospital outcomes between the two groups. The only major adverse cardiac event observed in that period was enzymatic (non Q wave) MI (two in the BMS cohort and one in the DES group, p=0.8). Complete long term (> 1 year) follow-up data was obtained for 98.7% of these patients at a mean follow-up time of 2.6 ±1.2 years (median of 3.2 years, ranging from 1.1 to 6.0 years). 92.6% and 86.3% of the patients in the BMS ISR and DES ISR groups were free of any MACE at six years (p<0.001, Figure 1).
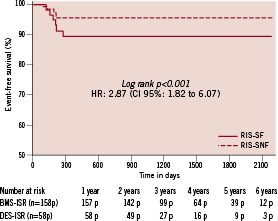
Figure 1. Major adverse cardiac events in BMS ISR and DES ISR groups.
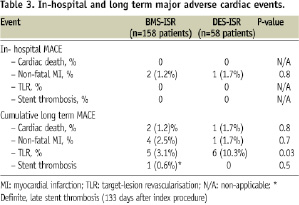
Of note, all the events occurred within the first 12 months. Although the occurrence of cardiac death, MI and stent thrombosis did not statistically differ between the cohorts, patients in the DES ISR group had significantly more recurrence of ischaemia-driven TLR during the follow-up period (10.3% vs. 3.1%, p=0.03). Notably, there was a single case of stent thrombosis among all the included patients, in the BMS ISR population. (definite, late thrombosis, 133 days after the index procedure).
Discussion
The main findings of this analysis are that percutaneous treatment of BMS and DES ISR with the deployment of another DES represents a simple, feasible and safe approach with high rates of acute success and relatively low incidence of serious adverse events in the long term follow-up. Even so, recurrence of restenosis among patients with previous DES ISR remains elevated (>10%), meaning that alternative solutions are still required.
The advent of BMS markedly reduced rates of acute recoil, abrupt vessel closure and chronic negative remodelling – the main mechanisms behind balloon-angioplasty failure. However, it soon became clear that the deployment of those devices could lead to an exacerbated local “healing” response resulting in an abnormal neointimal tissue proliferation within the stent and recurrence of ischaemic symptoms requiring additional revascularisation procedures. This phenomenon is observed in up to 30% of the cases according to patient and lesion complexity.
Prior to the introduction of DES, a wide variety of percutaneous approaches were tested to treat BMS ISR. Among them, intravascular brachytherapy (IVB) was the most successful alternative, with pivotal clinical trials showing promising middle term results9-11. Conversely, the widespread use of IVB was limited due to logistic reasons (need of a complex apparatus to its execution), radiation safety concerns, and evidence of late loss of clinical efficacy (the “late catch-up” phenomenon)12 and safety (late stent thrombosis)13.
The marked reduction of restenosis achieved with DES in the treatment of de novo lesions soon prompted physicians to apply this novel technology for the treatment of BMS ISR. As a consequence, the use of DES was tested against and showed superiority over balloon-angioplasty alone14, implant of another BMS15 and IVB16,17. Of note, the SISR trial randomised 384 patients with BMS ISR to either DES (Cypher™) or IVB16. At the end of nine months, target vessel failure was observed in 21.6% of the patients treated with IVB and 12.4% in those treated with DES (p=0.02). In the same way, the TAXUS-V trial compared 196 patients with BMS ISR treated with Taxus™ to 201 patients treated with IVB17. At nine months, patients allocated to the Taxus™ arm experienced a reduction of 60% in the need for target-vessel revascularisation (p<0.05). As a result of these studies, use of DES became the first choice of treatment for BMS ISR. However, it is important to highlight that most of these trials limited their follow-up period to two years of the index procedure. More recently, Alfonso et al presented the very long term results of the RIBS II trial comparing Cypher™ stent to balloon-angioplasty for the treatment of BMS-ISR. At four years follow-up, 76% of the patients treated with DES and 65% of those submitted to balloon-angioplasty (p=0.019) were completely free of MACE and the use of Cypher™ was an independent predictor of event-free survival18. Also in 2008, Oliver et al published the results of a meta-analysis with 14 studies (3,103 patients) comparing DES to IVB for the treatment of BMS-ISR. Compared to IVB, the use of DES significantly reduced the rate of revascularisation (OR 0.51, 95% CI 0.36-0.71), MACE (OR 0.55, 95% CI 0.39-0.79) and binary restenosis (OR 0.57, 95% CI 0.40-0.81). However, the follow-up time was limited to nine months, precluding the assessment of safety and efficacy of this approach in the long term.
Following the increased use of DES in the most complex scenarios, DES ISR also became increasingly prevalent. Intuitively, the deployment of another DES to treat the ISR became a treatment option in many centres worldwide despite the lack of large randomised controlled trials attesting the safety and efficacy of this strategy. Lemos et al were the first to report the nine month results of 24 patients with DES-ISR treated with Cypher™. They observed a recurrence of restenosis in 18.2% of the cases20. Later, Cosgrave et al evaluated the impact of switching the type of DES in the outcomes of patients with DES ISR21. In their analysis, 107 individuals received the same type of DES and 94 received a DES different from the one used in the index procedure. TLR occurred in roughly 16% of the entire cohort with no evidence of benefice of one strategy over the other. More recently, Steiberg et al reported from the Washington Hospital Center, (Washington, DC, USA) their experience with DES for the treatment of ISR22. One hundred nineteen patients with BMS ISR were matched to 119 patients with DES ISR, all treated with another DES deployment. At the end of one year, patients at the DES ISR group experienced significant more TLR (22.2% vs. 10.3%, p=0.01) with no significant difference regarding death and MI. Nevertheless, their follow-up was limited to the first year of the PCI.
Compared to the previous reported series and considering the extended follow-up period of our registry (the longest available so far), the overall rate of events among our patients is considerably inferior to what has been reported in these scenarios, especially for the treatment of DES ISR (<11%). Also notable are the absence of stent thrombosis in that cohort and the extremely low rate of thrombosis among patients with DES for a BMS ISR (a single case related to clopidogrel discontinuation due to GI bleeding). In favour of our casuistic, there is the fact that all patients were treated for their first ISR and none of them had been previously submitted to IVB.
Limitations
Although the DESIRE registry encompasses patients prospectively enrolled and followed-up, the present analysis with ISR patients is retrospective and therefore carries all the potential limitations of such method. The limited number of patients enrolled, especially in the DES ISR group, may play a role in the final results. Furthermore, the operator’s decision about the type of DES used to treat the ISR precludes any comparison between these devices. Finally, due to the small numbers, no predictors of failure after treatment of ISR could be identified. Thus, this paper has not the power to identify causes of DES failure for the treatment of ISR.
Conclusions
With the advent of DES, percutaneous treatment of both BMS and DES ISR became simple and safe, with relatively low rates of MACE and stent thrombosis in the very long term clinical follow-up.
Nevertheless, patients with previous DES restenosis are at significantly higher risk of presenting TLR in the FU when compared to those who received a DES for a BMS ISR. Specifically for those patients, alternative approaches such as IVUS guided PCI, use of a new generation of DES or drug-coated balloons should be tested in order to reduce ISR recurrence.

