Abstract
Aims: Treatment of in-stent restenosis (ISR) was historically considered the Achilles heel of percutaneous coronary intervention (PCI) and has been associated with worse clinical outcome than PCI of de novo lesions. However, comparative data on ISR and de novo lesions using drug-eluting stents (DES) are scarce. Therefore, we aimed to assess the impact of ISR on procedural and long-term outcome in patients treated with DES.
Methods and results: We analysed data from 5,144 patients enrolled in the prospective multicentre German Drug-Eluting Stent Registry (DES.DE). The registry included 872 patients (17%) treated for ISR with follow-up data (median 12.4 months) available for 817 patients (94%). Of the ISR patients, 37.1% (n=323) presented with acute coronary syndromes. In total, 1,027 DES were used (528 sirolimus-eluting stents and 499 paclitaxel-eluting stents), with successful implantation in 97.7% of patients. In the ISR cohort, myocardial infarction (MI) during hospitalisation was observed in 1.6% of patients (n=14) and in-hospital mortality was only 0.3% (n=3). Major adverse cardiac and cerebrovascular events (MACCE) rate at follow-up (defined as a composite of death, MI and stroke) was 8.7% (n=71) versus 8.2% (n=325) in patients treated for de novo lesions (p=0.63). Target vessel revascularisation (TVR) rate was 12.7% (n=100), numerically higher than in patients with de novo lesions (10.5%, p=0.07). Ten patients (1.3%) suffered from ARC definite stent thrombosis versus 0.7% observed in patients with de novo lesions (p=0.13). After adjustment for differences in baseline characteristics, TVR rates were statistically higher in the ISR cohort (OR 1.27, 95%CI 1.01-1.61, p=0.04), while MACCE rates remained comparable (OR 1.10, 95%CI 0.83-1.44, p=0.51). The type of stent used (sirolimus vs. paclitaxel-eluting stent) did not impact the rate of MACCE, TVR or definite stent thrombosis at one year.
Conclusions: Results from this large prospective multicentre registry confirm that treatment of ISR with DES is effective and safe, with similar procedural outcome but slightly higher revascularisation rates at one year compared to patients treated for de novo lesions, with no differences in outcome between sirolimus- and paclitaxel-eluting stents.
Introduction
Coronary stents have revolutionised percutaneous coronary revascularisation, improved both short- and long-term outcomes, and have set the stage for coronary interventions in complex lesions. However, the success of bare metal stents (BMS) is limited by the development of in-stent restenosis (ISR) in 20 to 30% of patients1,2. Related to its unique pathology, ISR was initially perceived as a benign phenomenon leading to stable angina and rarely presenting with myocardial infarction. On the other hand, percutaneous coronary intervention (PCI) of ISR had unfavourable long-term prognosis due to high recurrence rates3, and different pharmacological and mechanical therapies for ISR revealed disappointing long-term results. With the recognition that a considerable proportion of patients with ISR present with an acute coronary syndrome4,5 the perception of ISR has fundamentally changed. In addition, treatment of ISR with drug-eluting stents (DES) has been associated with favourable long-term outcome6-10, and DES are currently considered the main therapeutic modality for treating BMS restenosis. Similar outcomes at six months were documented between patients treated for ISR and those treated for de novo lesions when sirolimus-eluting stents (SES) are used11.
However, comparative data about treating ISR and de novo lesions using DES are scarce and the impact of the type of DES used is not clear. Therefore, we aimed to assess the impact of PCI on ISR on procedural and long-term outcome in patients treated with DES (sirolimus and paclitaxel-eluting stents) utilising data from of the prospective multicentre German DES.DE registry.
Methods
Registry design and study population
The prospective multicentre German DES.DE registry was initiated in October 2005 as an observational real world registry by the “Deutsche Gesellschaft für Kardiologie” (DGK, German Cardiac Society), “Bundesverband Niedergelassener Kardiologen” (BNK, German Society of Cardiologists in Private Practice) and “Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte” (ALKK, The Working Group of Leading Hospital Cardiologists). In phaseI of the registry (October 2005 to October 2006), only the twoFDA approved DES, paclitaxel-eluting stents (PES) (Taxus™; Boston Scientific Corp., Natick, MA, USA) and sirolimus-eluting stents (Cypher™; Cordis Corp., Miami Lakes, FL, USA) met the quality criteria of the registry. It was intended to collect data from at least 2,000 Taxus™, 2,000 Cypher™ and 500 BMS patients at 98 German sites. In all cases, the interventional strategy including choice of stent, use of intravascular ultrasound and the choice of periprocedural adjunctive therapy was at the discretion of the responsible physician. Details of the registry have been described elsewhere12.
In the period between October 2005 and October 2006, a total of 6,384 patients were enrolled in phaseI of the registry. The current analysis is based on 5,144 patients who underwent PCI using DES. The cohort was divided into 872 (17%) patients with and 4,272 (83%) without ISR.
Data collection and follow-up
Data were collected via an internet platform by the “Institut für Klinische Kardiovaskuläre Forschung” (IKKF, Institute for Clinical Cardiovascular Research) of the German Cardiac Society. Written informed consent for processing data at the “Institut für Herzinfarktforschung” (IHF, Institute of Myocardial Infarction Research, Ludwigshafen) was required. Baseline clinical and angiographic characteristics, certain procedural and clinical in-hospital events were recorded for all enrolled patients. Paper-based clinical and health quality follow-up assessments were performed at 3, 6, 9 and 12 months after initial stent placement and group allocation. IKKF forwarded corresponding questionnaires to the patients and collected all data and sent them to IHF for statistical processing. In case of an event, the IHF contacted the patient or the referring hospital for the reports. All events were verified by charts review or by direct contact with attending physicians. If patients could not be reached, the local government registration office was contacted. If patients stopped responding during follow-up, additional telephone follow-up was performed. Complete one-year follow-up was obtained in 95.1%. Relevant events (but not routine angiography without intervention) were reviewed by a Critical Event Committee (CEC), and processed by the IHF. Failure to collect detailed documentation (<1%) of a revascularisation event (PCI or CABG) was considered a target vessel revascularisation (TVR). A query management was established for missing or unclear data. Announced source data verification was performed at 24 randomly selected hospitals, with comparison of the documented data with the hospital charts.
Definitions
The endpoints of the registry and of the current analysis were the occurrence of TVR and major adverse cardiac and cerebrovascular events (MACCE), defined as the composite of death (cardiac and non-cardiac), myocardial infarction and stroke at one year follow-up. Death was defined as all causes of mortality. Myocardial infarction was defined as ST-elevation myocardial infarction (STEMI; ST-elevation at least 1 mm in two or more limb leads, or at least 2mm in two or more contiguous precordial leads or development of new left bundle branch block on the ECG) or non-ST-elevation myocardial infarction (NSTEMI; pathological increase of cardiac specific enzymes with CK-MB >1.5times of normal limits, TroponinT or I >99th percentile of normal value). TVR was defined as a repeated procedure, either PCI or CABG, on the target vessel. It should be noted that the definitions for major adverse cardiac events and MI are not uniform among the different clinical trials. In anumber of major adverse cardiac events definitions, different types of death (either cardiac or total death rate) and revascularisation parameters such as target vessel revascularisation have been used. Because the use of different definitions of major adverse cardiac events can cause confusion when comparing rates between trials, the steering committee decided to use only major adverse cardiac and cerebrovascular events as defined in the present report. Routine angiography was not part of the protocol in DES.DE for any subgroup of patients; therefore, all re-interventions are considered clinically driven. Definite stent thrombosis (presence of angiographic thrombus with a complete occlusion) was defined as proposed by the Academic Research Consortium (ARC)13.
Statistical analysis
Statistical analysis was performed using the SAS-statistical package, version 9.1 (Cary, NC, USA). Demographic characteristics, pre-existing risk factors, procedure-related variables, and one-year outcomes were summarised using mean value with standard deviation or median and interquartile range for continuous variables, and frequency and percentage for categorical variables. Differences in baseline, procedural, and angiographic characteristics, in-hospital and follow-up data were compared between patients with ISR and those with de novo lesions by Chi-square test, while continuous variables were compared by Wilcoxon rank sum test. The one year event-free survival rates for MACCE and TVR were analysed using Kaplan-Meier curves and were compared using the log-rank test. p-values <0.05 were considered significant and were results of two tailed tests. Stepwise multivariate logistic regression analysis was used to estimate the adjusted odds ratios with 95% confidence intervals for MACCE and TVR at one year. The variables entered into the multivariate models were age (>75years), diabetes, hypertension, smoking, hyperlipidaemia, positive family history of coronary artery disease, previous myocardial infarction, atrial fibrillation, STEMI, target vessel=left anterior descending, chronic total occlusion, long lesion (>15mm), type C lesion, bifurcation lesion and stent type.
Results
Baseline clinical characteristics
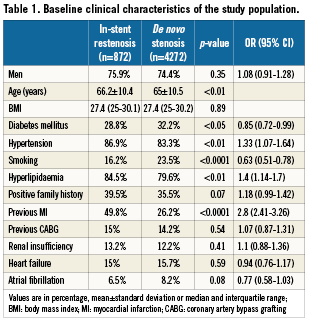
Data were extracted from 5,144 patients treated with 6,234 DES. At baseline, the 872 (17%) patients treated for ISR were older, had more commonly hypertension and hyperlipidaemia, and had more often history of previous myocardial infarction compared to 4,272 (83%) patients treated for de novo lesions. On the other hand, other risk factors such as diabetes mellitus and smoking were less common in the ISR group. Other clinical characteristics were equally distributed between both study groups as listed in Table 1.
Cardiac characteristics and angiographic status
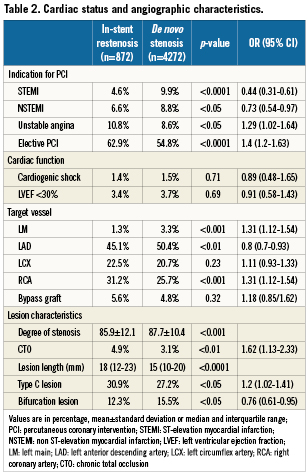
In the cohort of ISR, 37.1% (n=323) of patients presented with an acute coronary syndrome, compared to 45.2% (n=1,929) of patients with de novo lesions. Unstable angina was more dominant in the ISR group (10.8% vs. 8.6%, respectively), while STEMI dominated in the non-ISR group constituting 11.8% compared to 5.4%. Patients with ISR had angiographically more complex lesions with more frequent chronic total occlusions, type C lesions as well as long lesions. Details are listed in Table2.
Procedural characteristics
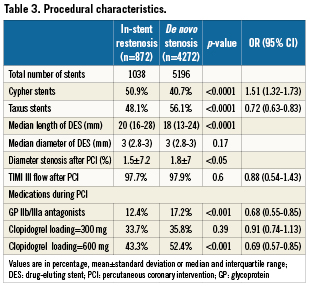
In total, 1,027 DES (528 sirolimus-eluting stents and 499 paclitaxel-eluting stents) were used in patients with ISR, with successful implantation in 97.7% of patients, compared to a 97.9% angiographic success rate in patients treated for de novo lesions (p=0.6).
Patients with ISR more commonly received SES, while patients with de novo lesions received more PES. The use of glycoprotein IIb/IIIa inhibitors and a higher clopidogrel loading dose (600mg) were more common in the group of patients treated for de novo lesions, probably explained by a higher number of acute coronary syndromes. Other procedural details are listed in Table3.
In-hospital outcome
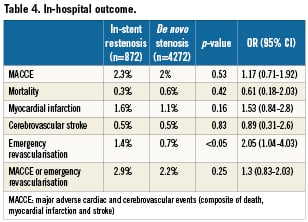
In-hospital major adverse cardiac and cerebrovascular event (MACCE) rate was 2.3% in patients with ISR compared to 2% for patients with de novo lesions (p=0.53). Though emergency revascularisation was more often performed in patients with ISR (1.4% vs. 0.7%, p <0.05), the overall incidence of MACCE and emergency revascularisation revealed no statistical difference between both patient cohorts. Data of the in-hospital outcome are listed in Table4.
One year outcome
The one year follow-up rate was 94% for patients with ISR and 93.5% for patients treated for de novo lesions (Table5). MACCE rates at one year were similar (8.7% in patients with ISR versus 8.2% in patients with de novo lesions, p=0.63). While mortality (3.5% versus 4.2%) and cerebrovascular stroke (0.8% versus 1.2%) tended to be lower after ISR, myocardial infarctions were significantly more often observed after treatment of ISR (4.7% versus 3.1%; p<0.05). The rates of TVR were 12.7% versus 10.5%, and ARC definite stent thrombosis occurred in 1.3% versus 0.7% of patients treated for ISR and de novo lesions, respectively. Though numerically higher in the ISR cohort, the latter differences did not reach statistical significance.
After adjustment for differences in baseline characteristics, TVR rates were slightly higher in the ISR cohort (OR 1.27, 95%CI 1.01-1.61, p=0.04), while MACCE rates remained comparable (OR 1.10, 95%CI 0.83-1.44, p=0.51). Determinants of TVR and MACCE at one year are listed in Table 6. Kaplan-Meier curves for event-free survival till one year of follow-up among both groups are shown in Figure1.
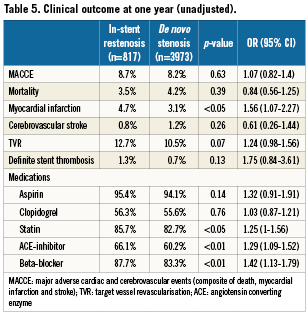
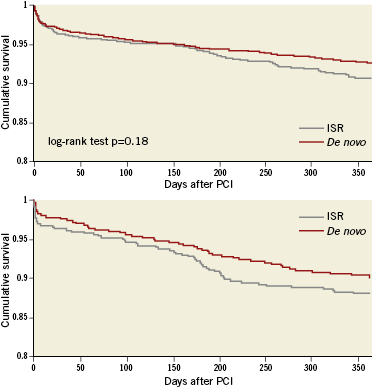
Figure 1. Kaplan-Meier curves for survival free of myocardial infarction/stroke and target vessel revascularisation up until one year of follow-up among patients with in-stent restenosis and de novo lesions.
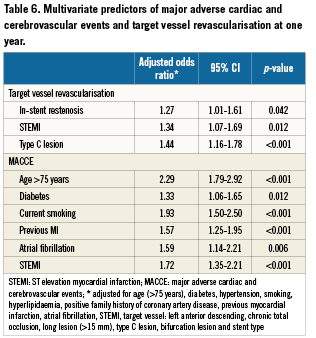
Patients treated for ISR were found more adherent to medications at one year follow-up. Statins, angiotensin converting enzyme inhibitors and ß-blockers were more frequently taken by patients with previous ISR, while adherence was similar for both aspirin and clopidogrel (Table5).
SES versus PES in ISR
Patients treated for ISR received more SES, while patients with denovo lesions received more PES. Patients treated with SES or PES were similar regarding baseline clinical and angiographic characteristics (Table7). Furthermore, the type of stent used (SES vs. PES) did not impact the rate of MACCE, TVR or definite stent thrombosis at one year follow-up. Data comparing the one year follow-up of patients with both types of DES used for ISR are presented in Figure2.
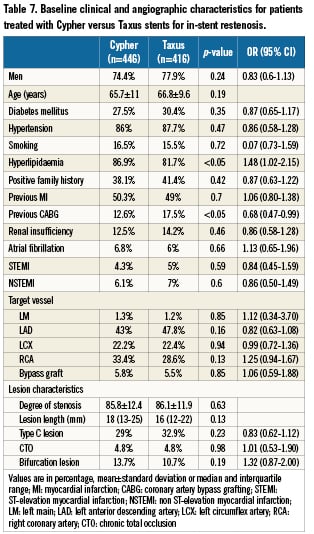
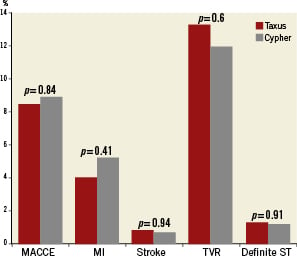
Figure 2. Clinical outcome at 12 months for patients treated with Cypher versus Taxus stents for in-stent restenosis. MACCE: major adverse cardiac and cerebrovascular events; MI: myocardial infarction; TVR: target vessel revascularisation; ST: stent thrombosis
Discussion
DES.DE is a large multicentre German registry characterised by ahigh follow-up rate and an excellent data quality, in addition to ahigh number of patients with “off-label” indications, probably allowing meaningful conclusions even from subgroups not extensively studied in randomised trials. ISR is such a subgroup, which has been traditionally considered as the “Achilles heel” of coronary stenting. The principal finding of the current analysis is that treatment of ISR with DES is both effective and safe, with similar procedural outcome but slightly higher revascularisation rates at one year compared to patients treated for de novo lesions. The outcomes did not differ between patients treated with sirolimus- or paclitaxel-eluting stents.
Patients with ISR are of particular interest because results of recent randomised controlled trials6-9 suggest a breakthrough for these patients with formerly unsatisfactory long-term results. A meta-analysis showed that both SES and PES effectively reduce the risk of recurrence with superior results compared to plain balloon angioplasty or brachytherapy10. The present analysis confirms that patients receiving DES for ISR face a reasonable 12 month TVR rate of 12.7%, but the event rate is still higher compared to patients with de novo lesions. Previous findings from the German Cypher registry in a large cohort of 1,511 patients treated with SES for ISR demonstrated acceptable TVR rates (9.3% at 6-months follow-up), that did not differ from patients treated for de novo lesions in the same registry (8.1%)11. Our results parallel these observations, and extend these findings to patients treated with both SES and PES and followed-up for one year. Despite the known differences in the underlying pathology for ISR and de novo atherosclerotic lesions, which were thought to necessitate different therapeutic modalities, ISR responds rather similar to de novo lesions when DES are being used.
Looking at safety endpoints at one year, overall mortality in both patient cohorts was similar, despite the fact that patients treated for ISR suffered more often from myocardial infarction. The increased incidence of myocardial infarction after treatment of ISR may be due to more advanced coronary artery disease or higher rates of stent thrombosis. In an analysis of 100 patients treated for ISR using SES, Le Feuvre et al14 reported a high risk of definite (4%) or probable (4%) SES thrombosis after four years. Conversely, the Tuscany Registry of Sirolimus for Unselected In-Stent Restenosis (TRUE registry) showed a cumulative incidence of stent thrombosis of 2.8% in 244 patients treated with SES for ISR at four years (seven events; 0.70% per year)15. Both studies, however, lack a valid control group. The reported occurrence of definite stent thrombosis at one year in the ISR patients in the current analysis was only numerically higher than that reported in patients with denovo lesions (1.3% vs. 0.7%), which needs further verification in larger observational studies. Obviously, differences in very late stent thrombosis (>1 year) cannot be evaluated from the current analysis.
In our registry, operators predominantly used SES for ISR, while PES were preferred for de novo lesions. This is most likely due to the fact that SES received the approval for treatment of ISR earlier than PES. The first generation SES are generally believed to induce superior neointimal suppression in comparison to PES. Previous studies reported the superiority of SES compared to PES particularly in complex lesions16,17. The ISAR-DESIRE study showed a trend towards a lower rate of angiographic restenosis and a significantly lower rate of TVR (8% vs. 19%) among ISR patients treated with SES compared to PES6. Such an advantage for SES over PES in the treatment of ISR is not supported by the present comparison. Whether newer generation DES would offer similar or better efficacy is currently unknown, and must await randomised controlled trials; yet, upcoming analyses from phases II and III of our registry comparing newer generation DES may further clarify this issue.
Recently, drug-eluting balloons have been suggested as an attractive modality for the treatment of ISR. In the PACCOCATH ISR trial, a significant reduction of ISR recurrence with a paclitaxel-coated balloon has been reported compared to plain balloon angioplasty18. Moreover, paclitaxel-coated balloon and paclitaxel-eluting stent showed similar outcome profile in a head-to-head comparison19. However, it appears that drug-coated balloons might be of benefit in treatment of ISR, but would be less effective in denovo lesions compared with DES20, the latter seem to be effective in both forms of disease.
Study limitations
The present analysis has the inherent limitations of any non-randomised multicentre registry and the findings should be regarded as hypothesis-generating. The registry findings may be limited by low rates of enrolment, under-reporting of events (e.g., stent thrombosis), as well as over-reporting of events (especially with death being defined as all causes of mortality), although reflecting the real world better than controlled randomised studies. Yet with enrolment of more than 6,000 patients, which were very closely monitored by two independent critical event committees, these problems were negligible. Second, there was no long-term follow-up and late events (beyond one year) may be missed. Late catch-up in general appears to be no problem with DES in randomised trials, but may occur in ISR. Third, the registry did not distinguish between DES-ISR and BMS-ISR, both have probably different pathophysiologies and hence outcomes may differ. Fourth, TVR dilutes potential differences of stent restenosis, because TVR in the non-stented segment would occur in a random manner, and thereby mask minor differences in TLR rates. However, angiography films were not centrally adjudicated, and there was no angiographic corelab for the registry, therefore, proper differentiation of TLR was not possible. On the other hand, TVR is more patient than lesion oriented, and remains a valid efficacy endpoint. Fifth, data about routine follow-up angiography (which is performed in some centres in Germany) have not been collected in the DES.DE registry. Sixth, data at one year follow-up showed that patients treated for ISR were more adherent to their medications compared to patients treated for denovo lesions, which might contribute in some way to the similar long-term outcome observed in both patient cohorts. Finally, data about emergency revascularisation and stent thrombosis should be interpreted with caution because of the low number of events and the lack of central angiographic adjudication.
Conclusion
This large contemporary real-world registry of DES revealed that treatment of ISR with DES is effective and safe, with similar procedural outcome, but slightly higher revascularisation rates at one year, compared to treatment with DES in de novo lesions; there were no differences between SES and PES.
Acknowledgement
We would like to express our thanks to the clinical research group at the Herz-Kreislauf Zentrum , Segeberger Kliniken GmbH, especially Mrs. Daniela Schuermann-Kuchenbrandt, Mrs. Kirsten Kassner, Mr. Guido Kassner and Mr. Marcus Ring.
This work was supported by Cordis Corporation (a Johnson & Johnson Company) and Boston Scientific Corporation.
Appendix
Organisation of the German DES.DE registry
Members of the Steering Committee: Christoph A. Nienaber (Chairman), Karl-Heinz Kuck (Chairman), Christoph Bode, Fokko de Haan, Gert Richardt, Georg Sabin, Jochen Senges, Sigmund Silber, Jürgen Stumpf, Ulrich Tebbe, Stefan Willich, Thomas Fetsch, Gabriele Sailer, Steffen Schneider
Members of the Clinical Event Committee: Bernhard Meier, Ahmed Khattab, Martin G Gottwik
Internet data acquisition: Institute of Clinical Research of the German Cardiac Society; Thomas Fetsch, Petra Kremer
Statistical analysis: Heart Center Ludwigshafen; Steffen Schneider, Matthias Hochadel
Conflict of interest statement
This work was supported by Cordis Corporation (a Johnson & Johnson Company) and Boston Scientific Corporation.
Christoph A. Nienaber and Gert Richardt received lecture fees from Cordis and Boston Scientific; both are consultants for both companies. Jochen Senges received lecture fees from Cordis. The other authors have no conflicts of interest to declare.

