
Treatment of in-stent restenosis (ISR) remains challenging and associated with worse late clinical outcomes compared with treatment of de novo lesions1-3. The widespread use of drug-eluting stents (DES) replacing bare metal stents (BMS) in everyday clinical practice helped to reduce the rates of clinical and angiographic ISR, especially in patients with complex clinical and angiographic characteristics. However, although rare, treatment of DES-ISR has proved to be more complex than that of BMS-ISR1. Virtually all possible therapeutic modalities for coronary intervention have been tested in patients with ISR1-3. Actually, some strategies with profound antirestenosis effects (i.e., brachytherapy) only gained clinical acceptance in this unique niche1. Currently, however, clinical practice guidelines only recommend the use of DES or drug-coated balloons (DCB) for patients with either BMS-ISR or DES-ISR (both strategies with a class I-A recommendation)3. Recently, many randomised clinical trials (RCT) have illuminated the field, providing robust clinical evidence supporting the safety and efficacy of DES and DCB in these patients4-11. However, the dilemma regarding whether to use second-generation DES or DCB in patients with ISR remains unsolved. Indeed, data from existing RCT comparing these therapeutic alternatives head-to-head may still be considered controversial. In most studies DES provide significantly better acute and long-term angiographic results regarding minimal lumen diameter and % diameter stenosis as compared with DCB2,5,6,10. However, the results of both strategies are largely similar when other well-accepted angiographic outcome measures, such as late lumen loss (LLL) or binary restenosis rates, are considered4-10. The larger acute gain systematically obtained by DES compared with DCB4-11 may be offset by a larger LLL during follow-up, according to the “the more you gain the more you lose” principle, that, in some studies8,9, remains operative despite the strong antiproliferative properties of DES. This is why the use of LLL to compare balloon- with stent-based procedures has been critically brought into question. From a clinical standpoint, however, both strategies are very safe and provide favourable long-term clinical outcomes4-11. Nevertheless, results remain consistently worse in patients treated for DES-ISR irrespective of the selected therapeutic modality1. Some studies indicate that, in these challenging patients with DES-ISR, new-generation DES are more effective than DCB for reducing the need for repeat revascularisation6.
Unfortunately, we still do not have clear clinical or anatomical clues to inform the decision-making process required to select between these competing therapeutic modalities in the individual patient. Intravascular imaging, and especially optical coherence tomography (OCT) with its unsurpassed spatial resolution, provides valuable insights regarding potentially correctable underlying mechanical factors in this anatomic setting1. Specifically, stent underexpansion plays a major role in many patients with ISR and therefore should be aggressively tackled. Moreover, OCT provides unique information on the underlying pathologic substrate causing the stent obstruction (neointimal hyperplasia versus neoatherosclerosis)1-3. Actually, it is hard to imagine that these completely distinct morphologic substrates would not play a significant role in the relative efficacy of the available interventions. Nevertheless, once again, we are currently still unable to use information on tissue characterisation to guide the selection of the optimal therapeutic strategy in a given patient1.
Repeat stenting with new-generation DES guarantees excellent acute and late angiographic and clinical results in this challenging scenario. However, many interventional cardiologists consider that the systematic implantation of an additional metal layer is not justified since equivalent late results may be obtained using DCB. Moreover, although unproved, the fear of prolonging the vulnerable period for vessel thrombosis with repeat DES implantation is put forward by some operators as a reason for selecting a DCB1-3.
This is why additional information from RCT comparing these novel competing therapeutic modalities head-to-head is more than welcome to enhance our understanding of their relative safety and efficacy.
Present study
In this issue of EuroIntervention, an RCT comparing a new DCB with a new-generation sirolimus DES in patients with ISR is presented by Jensen et al11.
The BIOLUX multicentre RCT (BIOtronik Clinical performance of the Pantera LUX paclitaxel-coated balloon versus the drug-eluting Orsiro hybrid stent system in patients with in-stent restenosis) allocated (2:1) 229 patients with either BMS-ISR or DES-ISR, to DCB (n=157) or DES (n=72) stratified by diabetic status11. DCB proved to be non-inferior to DES regarding in-stent LLL (primary efficacy endpoint): 0.03±0.40 mm in the DCB arm, 0.20±0.70 mm in the DES arm (p=0.40, non-inferiority p<0.0001). In addition, although the study was largely unpowered for clinical events, rates of target lesion failure (a composite of cardiac death, target vessel myocardial infarction and clinically driven target lesion revascularisation) were similar at one year (16.7% in the DCB arm and 14.2% in the DES arm, p=0.65) and at 18 months (19.5% versus 18.6%, respectively). The authors concluded that, in patients with ISR, this novel DCB provides similar LLL and TLF rates compared with a second-generation sirolimus DES.
This randomised study is of clinical interest as it compares two attractive new devices in patients presenting with any type of ISR. A novel paclitaxel DCB (using butyryl-tri-hexyl citrate as a carrier) was compared with another novel, ultra-thin, DES eluting sirolimus from a biodegradable polymer. Most RCT on patients with ISR selected an iopromide-based paclitaxel DCB (with robust evidence for efficacy on ISR) to be compared with a second-generation DES eluting everolimus from a permanent polymer (as this DES is considered a best in class comparator)4-10. Notably, however, in de novo lesions, the sirolimus DES with biodegradable polymer used in the present trial compared favourably with the reference standard DES eluting everolimus from a permanent polymer12.
The angiographic findings are of major interest. In contrast with most previous studies in the field, the present protocol aimed to exclude very short (<6 mm length) lesions. However, the lesions eventually treated were rather focal (median lesion length 4.6 and 4.8 mm in the DCB and DES arms, respectively). On the other hand, reference vessel diameter and both predilation balloons and final devices tended to be larger in the DCB arm. Nevertheless, the DES arm obtained a significantly larger acute gain, leading to a larger minimal lumen diameter and smaller residual stenosis immediately after the procedure. Surprisingly, although in this study a significantly larger acute gain was achieved in the DES arm and the LLL was similar with the two strategies, eventually minimal lumen diameter at follow-up was not different in the two arms. This differs from previous studies where for a similar LLL the larger acute gain obtained with DES translated into a larger minimal lumen diameter or reduced % diameter stenosis at late follow-up. Alternatively, in spite of data scattering, the DES arm obtained a significantly smaller in-stent % diameter stenosis at late follow-up (23.7±25.8 vs. 28.5±15.7%, p<0.001). In this regard, it is important to keep in mind that some previous landmark trials on ISR actually selected this angiographic parameter as the primary endpoint to compare DES vs. DCB7. Were this endpoint to have been selected in the current study, a valid interpretation would have been to suggest a potential benefit with the use of DES. Moreover, the differences between in-stent and in-segment % diameter stenosis found in this trial may be interpreted simply as the inability of DES to influence coronary segments outside their limits but also as the occurrence of a geographical miss during treatment.
From a purely methodological standpoint other issues should also be considered. Coronary angiograms were analysed centrally but the analysts were not blinded to the allocated treatment. In addition, late angiographic surveillance was obtained in <80% of patients in both arms, which may be criticised considering that the trial had an angiographic primary endpoint. Also, adjudication of clinical events was not blinded. This is unusual in a head-to-head randomised study and might have induced outcome ascertainment biases. Finally, the authors acknowledge that the sponsor was fully involved in the study design, data collection, monitoring, analysis (including the statistical analysis) and final interpretation. Notably, the two devices compared in this trial are manufactured by the same company.
Although in this trial the clinical results were largely consistent in patients with BMS-ISR (accounting for only one third of the population) and DES-ISR, numerically higher rates of target lesion revascularisation were observed with the two therapeutic strategies in patients presenting with DES-ISR.
Finally, the use of intracoronary imaging was not mandated by the protocol but could have provided valuable information regarding both the extent of stent underexpansion and the pathologic substrate of the culprit lesion. Actually, the use of intracoronary imaging is suggested by recent clinical practice guidelines to optimise final results in these patients3. In this regard, pressures used during repeat stenting were just moderate (mean 15.5 bar) and only half of the patients in this arm underwent post-dilation. Of note, post-dilation is usually not recommended after DCB due to the fear that drug might be detached from the vessel wall. Whether a more systematic or aggressive strategy to tackle underlying underexpanded stents could have improved the results of the study remains possible but completely speculative.
Final remarks
Both new-generation DES and DCB provide satisfactory long-term clinical and angiographic outcomes in patients with ISR. The BIOLUX trial provides new information on the relative value of two novel devices that may be incorporated into our existing armamentarium to treat ISR11. Figure 1 presents an ad hoc meta-analysis that includes the results of all currently available RCT comparing DCB with second-generation DES in this setting. We should humbly acknowledge that results of repeat interventions, especially in the growing subset of patients presenting with DES-ISR, remain poorer as compared with those obtained in patients with de novo lesions. Further studies are warranted (the “saga” must continue) to identify the best strategy for each individual patient and also how to optimise the results of currently available therapeutic modalities in this challenging anatomic scenario.
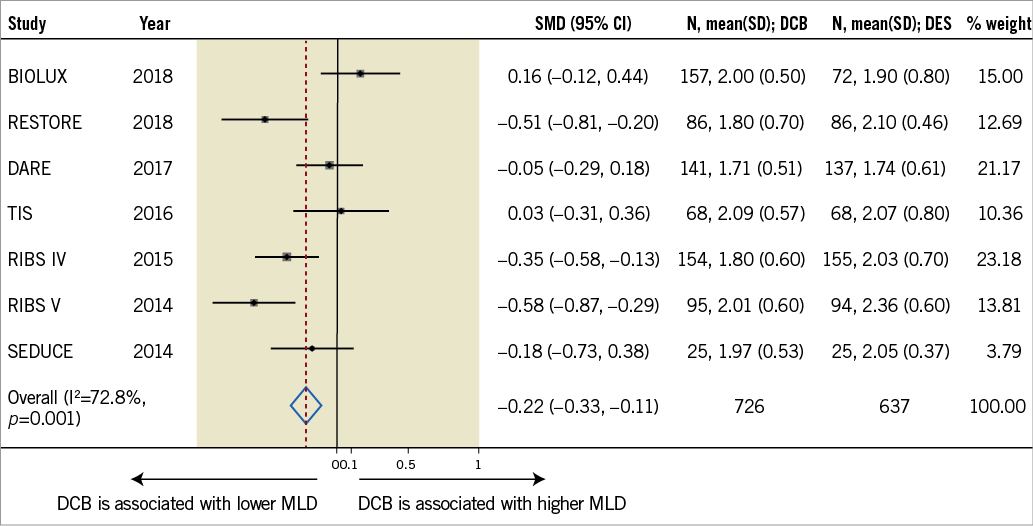
Figure 1. Meta-analysis of published RCT comparing DCB with second-generation DES (data from references 4-6, 8-11). Summary plot for in-segment minimum lumen diameter at late follow-up. The relative size of the data markers indicates the weight of the sample size from each study. CI: confidence interval; MLD: minimum lumen diameter; SMD: standardised mean difference
Conflict of interest statement
The authors have no conflicts of interest to declare.

