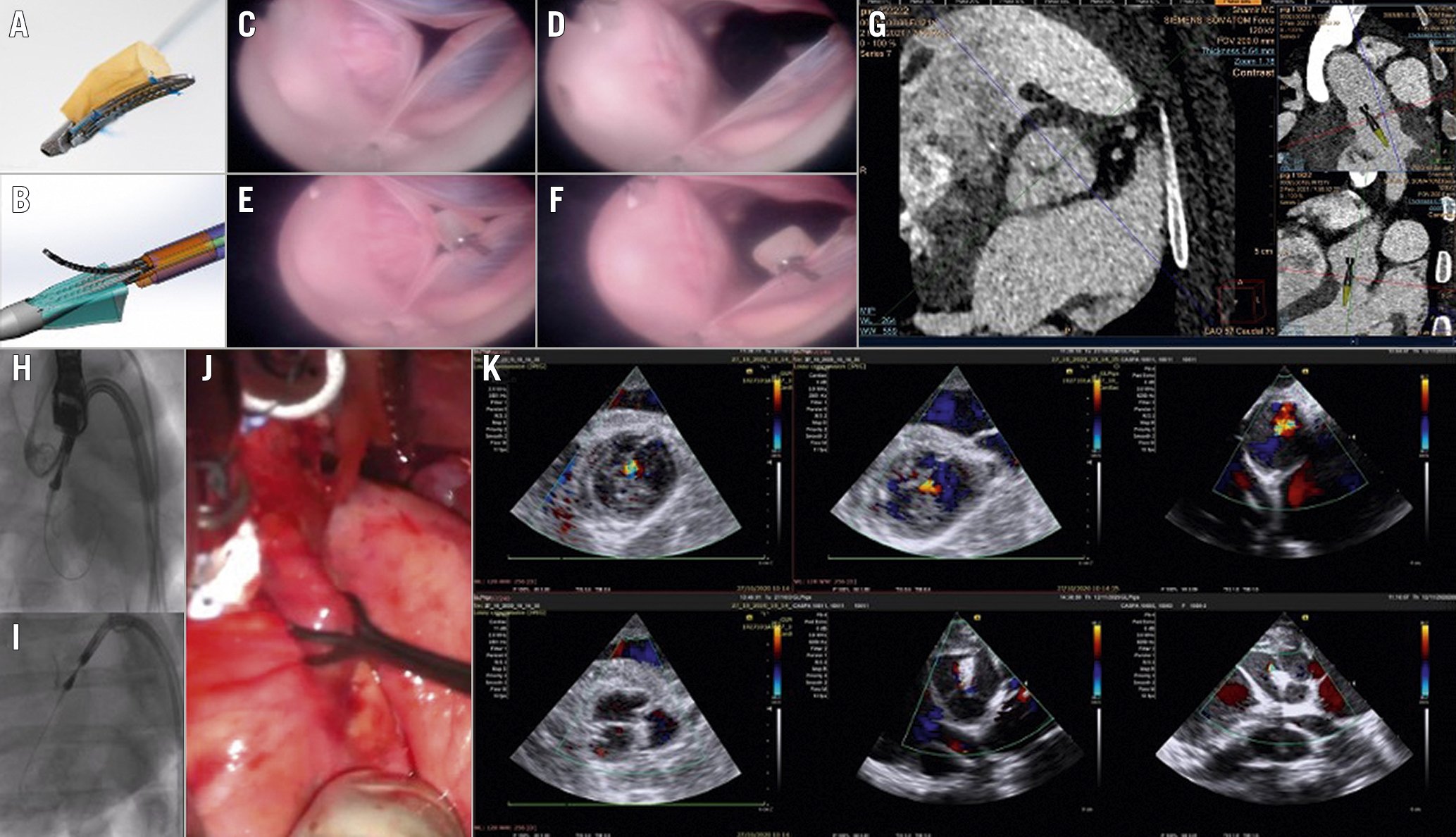
Figure 1. The Cusper preclinical proof-of-concept study. A) The Cusper device. B) Delivery system capsule. C-F) Ex vivo model: en face view of a porcine aortic valve with aortic regurgitation induced by aortic root dilatation with and without the device at systolic and diastolic phases. G) Computed tomography planning: on the left – an en face view of the aortic valve post annular alignment and positioning of the axis in an overlap (of the RCC and LCC) view. The upper right figure shows the implantation angle and the delivery capsule superimposed on the image to provide exact implantation depth. H-I are fluoroscopic images of a transcatheter delivery of the Cusper device in a pig. H) An image in an overlap (RCC and LCC) view of an open grasping arm following capsule retraction prior to implantation in the NCC. I) The Cusper is fully deployed and functioning following the release of the proximal and distal fixations and the retrieval of the delivery device and guidewire. J) Aortic regurgitation model: pulling at a constant 6 Newton force on the porcine aortic root induced a transient in vivo aortic regurgitation. K) The upper 3 short axis epicardial Doppler images demonstrate moderate to severe aortic regurgitation achieved by pulling on the porcine aortic root of 3 animals at a force of 6 Newtons and the lower 3 figures show the corresponding Doppler short axis images post Cusper implantation during the application of the same force. LCC: left coronary cusp; NCC: non-coronary commissure; RCC: right coronary cusp
Surgical aortic valve replacement (SAVR) is currently the recommended treatment of aortic regurgitation (AR)1. An alternative approach is transcatheter aortic valve implantation (TAVI), however, this procedure is reported to have higher than acceptable procedural complications and mortality rates2. The JenaValve (JenaValve Technology), a self-expanding valve with three integrated locators, has been tested in patients with native primary AR234. It is available in Europe and is currently being evaluated in the pivotal ALIGN-AR study (ClinicalTrials.gov: NCT04415047).
We report on a proof-of-concept study assessing a novel device, Cusper (CUSPA), for transcatheter aortic valve repair (TAVR) for AR.
The Cusper device is made of a nitinol clip (3.4 mm x16 mm) and pericardial sac (6 mm diameter) attached to the clip (Figure 1A). The implant is crimped in a dedicated delivery catheter with a proximal and distal fixation holder (Figure 1B). The system is configured for percutaneous implantation through a 16 Fr sheath. The Cusper is designed to fill the effective regurgitant orifice (ERO) of the aortic valve.
The proof-of-concept was performed using 3 methods. First, bench tests were performed using an ex vivo model of a pig heart connected to a pulsatile pump at the left ventricular apex. A fibre optic device was inserted through the right carotid artery. The aortic root was manually dilated to produce AR. The Cusper device was delivered through a delivery system to one of the cusps (Figure 1C-Figure 1F, Moving image 1). The Cusper filled the ERO almost entirely.
Second, an in vivo transcatheter implantation of the Cusper was performed in 9 healthy pigs assisted by computed tomography planning (Figure 1G). The experiment protocol was approved by the animal study committee of the Assaf Harofeh Medical Center, Israel and the Lahav Research Institute, Israel. Figure 1H-Figure 1I and Moving image 2 show an example of the fluoroscopy of the Cusper in the delivery system pointing to the non-coronary cusp (NCC) prior to and after being released. Overall, 7 devices were successfully implanted in 7 animals; 2 procedures failed. Embolisation, perforation and distortion were part of the learning curve of the implantation procedure.
Finally, an efficacy proof-of-concept study was performed in 3 AR model pigs. The aortic root was exposed and pulled at a force of 6 Newtons at the NCC - right coronary cusp and NCC - left coronary cusp commissures using a force gauge to produce transient moderate to severe AR (Figure 1J, Figure 1K, upper figures). The AR was reassessed in these pigs with Doppler echocardiography following implantation of the Cusper device. Re-induction of AR using the same force did not produce more than trivial AR after implantation of the device (Figure 1K, lower figures).
The JenaValve is the most advanced transcatheter device for AR on the regulatory pathway; however, when taking into consideration its current structure and the nature of valvular diseases, we assume that multiple devices will be needed for broad coverage of the different pathologies associated with AR. We demonstrated a proof-of-concept of the Cusper device which may offer a novel mechanism for transcatheter treatment of AR, and thus may theoretically broaden the future transcatheter armamentarium of structural interventions for AR patients.
Long term animal and human cadaver studies are currently underway.
Conflict of interest statement
Y. Feld is the founder and director of Cuspa Ltd., Paragate Medical Ltd., and CorAssist Cardiovascular Ltd. A. Weigler is CEO of Cuspa Ltd. D. Yakobi is a consultant to Cuspa Ltd., but has not received any payment for the preparation of the manuscript. S. Hazan is an employee of Cuspa Ltd.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Ex vivo video of the Cusper device in a porcine aortic valve.
Moving image 2. Fluoroscopic video of transcatheter delivery of the Cusper device.

