
Abstract
Aims: The aim of this study was to assess the safety and performance of the ACURATE neo transcatheter heart valve and its transfemoral delivery system.
Methods and results: The prospective, multicentre “CE-approval cohort” consists of a prospective series of the first 89 patients implanted with the ACURATE neo prosthesis. The primary endpoint was all-cause mortality at 30 days. Mean patient age was 83.7±4.4 years and logistic EuroSCORE I was 26.5±7.7%. Procedural success was obtained in 84 patients (94.4%). At 30 days, three patients had died, and two major strokes and one reintervention for a ventricular septal defect occurred, leading to a major adverse cardiac and cerebrovascular event (MACCE) rate of 6.7%. Eight patients (10.3%) received a permanent pacemaker. At one year, 20 patients (22.5%) had died and the MACCE rate was 27%. Effective orifice area was 1.76±0.34 cm2, and mean gradient 7.5±2.8 mmHg. Only three patients (4.5%) had moderate paravalvular regurgitation. NYHA Class III/IV was present in 94.4% of patients at baseline, in 9.9% at 30 days and in 4.5% at one year post procedure.
Conclusions: This first-in-human experience with a novel self-expanding heart valve showed low rates of procedural mortality, major stroke and pacemaker implantation, and good performance outcomes.
Abbreviations
EOA: effective orifice area
MACCE: major adverse cardiac and cerebrovascular events
NYHA: New York Heart Association
TAVI: transcatheter aortic valve implantation
Introduction
Transcatheter aortic heart valve implantation (TAVI) has become an established treatment option for high-risk patients with aortic stenosis. Randomised controlled trials with early-generation self-expanding and balloon-expandable devices have shown non-inferiority or even superiority in comparison to surgical aortic valve replacement, but were associated with higher rates of new pacemaker implantations and moderate to severe paravalvular regurgitation, the latter being associated with an increased mortality risk1-3.
Newer-generation devices have been developed to address these limitations, such as the self-expanding ACURATE TA™ prosthesis (Symetis SA, Ecublens, Switzerland) for transapical delivery. The prosthesis has specific design features to allow easy and intuitive implantation and has shown low pacemaker and paravalvular regurgitation rates in clinical trials and registries4,5. The encouraging results led to the development of the ACURATE neo™ prosthesis (Symetis) (Figure 1), translating the key features of the ACURATE TA into a transfemoral platform. Specifically, flexible stabilisation arches allow axial self-alignment, the upper crown allows supra-annular anchoring and captures the native leaflets, hence mitigating the risk of coronary obstruction and paravalvular regurgitation, the lower crown has minimal left ventricular outflow tract protrusion, hence avoiding interference with the conduction system, and the pericardial skirt acts as a seal against paravalvular regurgitation. Additionally, the BioFix™ (Symetis) anticalcification treatment will support the long-term durability of the valve. The ACURATE neo prosthesis has supra-annular leaflets, which allow a large effective orifice area (EOA) and low gradients. With the exception of the CoreValve® Evolut™ R prosthesis (Medtronic, Minneapolis, MN, USA), other transcatheter aortic bioprostheses are placed intra-annularly. The ACURATE TF delivery system is compatible with 15 Fr expandable sheaths and one 8 Fr rigid sheath and has a very flexible shaft facilitating easy tracking even in tortuous aortic anatomy6,7.

Figure 1. The ACURATE neo prosthesis. Stabilisation arches allow axial self-alignment of the prosthesis, the upper crown allows supra-annular anchoring and the lower crown is designed for minimal protrusion in the left ventricular outflow tract. The skirt acts against paravalvular leakage.
It was the aim of the “CE-approval cohort” study to assess the safety and performance of this novel device in high-risk patients treated for severe aortic stenosis. Data of this cohort led to CE certification in Europe in September 2014.
Methods
STUDY DESIGN AND POPULATION
The study population consisted of the first 89 patients treated with the ACURATE neo prosthesis from January 2012 to October 2013, prior to its commercialisation.
The main inclusion criteria were: severe aortic stenosis characterised by a mean aortic gradient >40 mmHg, or peak jet velocity >4.0 m/s, or aortic valve area of <1.0 cm2, age ≥75 years, logistic EuroSCORE I ≥20%, New York Heart Association (NYHA) functional Class >II, aortic annulus diameter ≥21 mm and ≤27 mm, and signed informed consent. The main exclusion criteria were: unicuspid or bicuspid aortic valve, extreme eccentricity of calcification, severe mitral regurgitation (>2+), pre-existing prosthetic heart valve or ring, left ventricular ejection fraction <30%. The full list of inclusion and exclusion criteria is provided at clinicaltrials.gov #NCT03003650. Follow-up occurred at discharge or seven days (whichever occurred first), at 30 days and one-year post procedure and included NYHA classification, National Institutes of Health Stroke Scale and mini mental state exam assessments, blood draw and echocardiography. Patients were not regularly assessed by a neurologist.
The study was conducted according to the Declaration of Helsinki, and approved by the local ethics committees and competent authorities; all patients provided written informed consent prior to any study procedure. No echocardiographic core laboratory was used. Except for the first-in-human cohort and the Japanese cohort, monitoring included 100% source document verification, and an independent clinical events committee adjudicated all deaths and major adverse cardiac and cerebrovascular events (MACCE).
STUDY DEVICE AND PROCEDURE
The ACURATE neo aortic bioprosthesis with its ACURATE TF delivery system has been described previously6,7. In brief, it has a self-expanding nitinol frame with porcine pericardial leaflets in the supra-annular position. In order to obtain stable positioning and anchoring together with some self-alignment, the valve opens from top to bottom, unsheathing first the upper crown, then the stabilisation arches and, in a second step, the lower crown to anchor the device inside the annulus. The prosthesis is partially repositionable. The ACURATE neo prosthesis is available in sizes S, M, and L, which correspond to diameters of 23, 25 and 27 mm, respectively, and allows treating aortic annulus diameters from 21 to 27 mm. At the time of the study, the system was used with either an 18 Fr or a 20 Fr introducer sheath. Predilatation was mandatory and post-dilatation was left to the discretion of the implanting physician. The recommended medication regime was acetylsalicylic acid lifelong, and dual antiplatelet therapy with clopidogrel 75 mg/day for six months post procedure.
ENDPOINTS AND DEFINITIONS
The primary endpoint was all-cause mortality at 30-day follow-up. Secondary endpoints were: MACCE at 30 days and at one year, defined as death, myocardial infarction, reintervention and stroke; functional improvement from baseline as per NYHA functional classification at 30 days and one year; procedural success defined as complete deployment, normal coronary perfusion, paravalvular or central leak
STATISTICAL ANALYSIS
No formal sample size calculation was conducted. Patients who received a prosthesis other than the study valve (e.g., conversion to open heart, valve-in-valve) were excluded from echocardiographic follow-up, but included in safety assessments. For quantitative variables, means and standard deviations (SD) were calculated, and for categorical data absolute and relative frequencies. The Wilcoxon signed-rank test and paired t-test were applied for comparison; p-values of <0.05 represent significance. Data analysis was performed using SAS, Version 9.3 (SAS Institute, Cary, NC, USA).
Results
The “TF 89 cohort” consisted of the first 89 patients implanted with the ACURATE neo prosthesis. Clinical follow-up compliance at 30 days and one year was 100%.
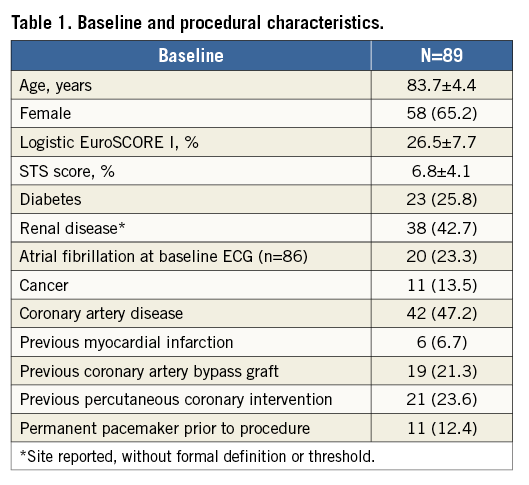
Patients were 83.7±4.4 years old with a logistic EuroSCORE I of 26.5±7.7% and an STS score of 6.8±4.1%. Patients had several comorbidities; nearly half of the patients had coronary artery disease (47.2%), renal disease (42.7%), or arrhythmias (41.6%) (Table 1). The procedure was performed under general anaesthesia. Predilatation was performed in all but one patient; 34 patients (38.2%) received a prosthesis size S, 35 (39.3%) size M and 20 (22.5%) size L. Post-dilatation was performed in 43 patients (48.3%). Time from femoral insertion of the delivery system to withdrawal post implant was 6:32±2:50 minutes, and the total procedure time was 66±28 minutes. The procedure was performed successfully in 84 patients (94.4%). In three patients, a valve-in-valve procedure was required (two for dislodgement during retrieval with subsequent suboptimal implantation height and one for a low implant), two of whom received a Medtronic CoreValve prosthesis and one an Edwards SAPIEN XT prosthesis (Edwards Lifesciences, Irvine, CA, USA). One aortic rupture due to post-dilatation occurred, which was detected post procedure; the patient subsequently died. One conversion to surgery occurred due to leaflet damage of the prosthesis following overinflation of the valvuloplasty balloon during post-dilatation. This patient died on day 2 post procedure. During enrolment, the delivery system was optimised to facilitate positioning and simplify retrieval. The last 34 patients were treated with the novel device iteration; none of them experienced a valve-in-valve procedure. No coronary obstruction or impairment of the mitral valve was observed.
All-cause mortality at 30 days was 3.4% (n=3). Aside from the two deaths mentioned above, the third death at 30 days was caused by a third-degree AV block. Further, two major strokes and one reintervention for a ventricular septal defect occurred, leading to a MACCE rate of 6.7% (Table 2). The ventricular septal defect was treated with reconstruction of the myocardium and pericardial patch which required the prosthesis to be removed and replaced by a surgical aortic valve on day 17 post procedure. The patient was discharged in a stable condition 13 days later. Excluding patients with prior pacemaker, the 30-day pacemaker rate was 10.3% (8/78). Between 30 days and one year, 17 additional deaths occurred. Causes were unknown in two, sudden death in two, neurological events in two, and cardiac decompensation in one patient, infection/sepsis in five patients, respiratory events in two patients, and trauma, cancer, and renal failure in one patient each.
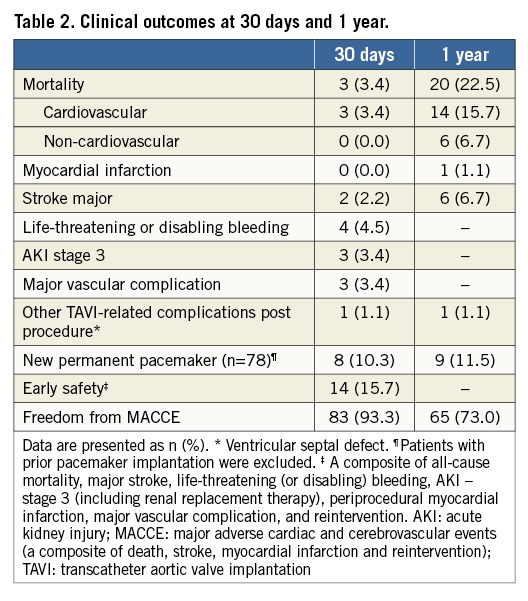
Follow-up echocardiography data were available for 82 patients at 30 days and for 66 patients at one year. The EOA improved from 0.67±0.17 cm2 preoperatively to 1.77±0.32 cm2 at 30 days and remained constant at one year (Table 3, Figure 2). Moderate paravalvular regurgitation was observed in four patients at 30 days and in three patients at one year. NYHA class improved in nearly all patients, with 95.5% of patients being in NYHA Class I or II at 12 months (Figure 3).
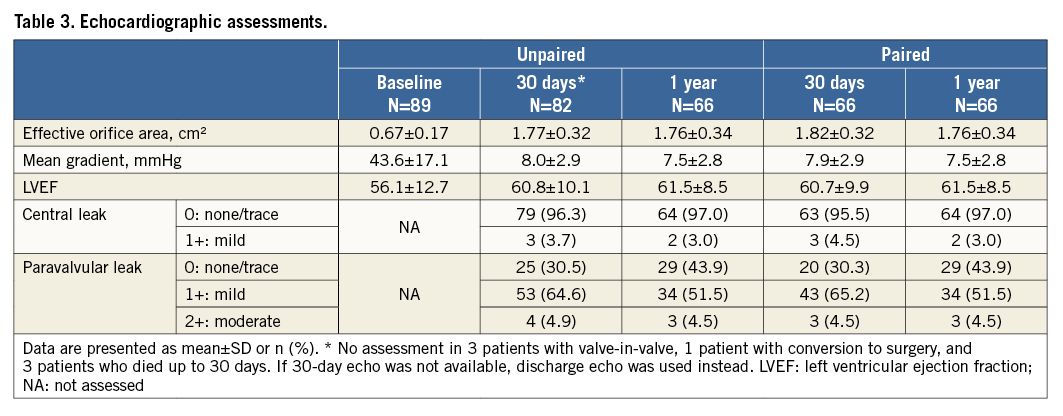
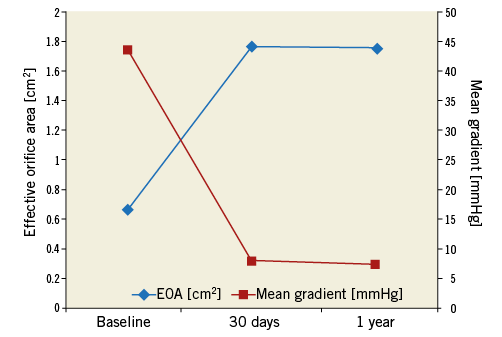
Figure 2. Echocardiographic parameters at baseline and follow-up. Data are available for 82 patients at 30 days and 66 patients at one year. The EOA increased significantly from baseline to 30 days (p<0.0001); the mean gradient decreased significantly (p<0.0001). Values remained stable between 30 days and one year (p=0.24 for EOA and p=0.28 for mean gradient). EOA: effective orifice area
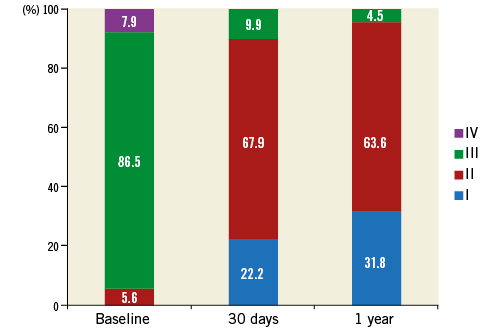
Figure 3. NYHA classification at baseline, 30 days and one year. NYHA class improved significantly from baseline to 30 days (p<0.0001); no significant difference was observed from 30 days to one year (p=0.307). NYHA: New York Heart Association
Discussion
The main finding of this CE-approval cohort of 89 patients is that transfemoral TAVI with the novel ACURATE neo transcatheter heart valve and its delivery system is safe and effective. Even though this series (a) included an elderly high-risk population with an average age of 83.7 years and a logistic EuroSCORE I of 26.5%, (b) included first-in-human use and best practices on how to implant this prosthesis via the transfemoral approach needed to be established, and (c) included device iterations, outcomes are very good and comparable to other contemporary transcatheter heart valve prostheses. The encouraging results in this early phase are most likely attributed to the self-seating, self-sealing design of the prosthesis, as the top-down deployment leads to a stable, easy implantation in the correct anatomic position6,7.
This is particularly important as previous experiences gained from first-in-human use or with similar devices were available for devices such as the the CoreValve Evolut R9,10, the Direct Flow Medical valve (Direct Flow Medical, Santa Rosa, CA, USA)11, the LOTUS™ valve (Boston Scientific, Marlborough, MA, USA)12, the Portico™ valve (St. Jude Medical, St. Paul, MN, USA)13,14, and the SAPIEN 3 prosthesis (Edwards Lifesciences)15. The total procedure time of 66±28 minutes with 6:32±2:50 minutes from time of femoral insertion of the delivery system to withdrawal post implant underlines the intuitive use of the device. In contrast, procedure times around 1.5 hours were reported for several novel next-generation devices, such as for the Direct Flow Medical multicentre experience16, the CoreValve Evolut CE-mark study9, and the REPRISE II study17.
Three valve-in-valve procedures due to dislodgement during retrieval and malpositioning occurred. As a matter of fact, the risk of valve migration during retrieval of the delivery catheter has been an issue for most self-expanding TAVR devices; however, the final versions of the ACURATE neo and its ACURATE TF delivery system appear to have adequately addressed this issue7; positioning became easier and no valve-in-valve procedure occurred in the last 34 patients enrolled.
The mortality of 3.4% at 30 days is lower than in first-generation devices with a pooled 30-day mortality of 7.8% (1.7-14.3%)18 and comparable to recent reports of next-generation devices ranging from 0% to 4.2%9,15-17,19-21. Moreover, two of the three deaths occurred due to intraprocedural complications which are expected to diminish with the updated delivery system and increased operator experience with this device. In contrast, the one-year mortality appears to be higher than in recent studies. Notably, complications associated with increased mortality such as paravalvular regurgitation, acute kidney injury, periprocedural myocardial infarction and major stroke22,23 were low so that it is likely that the one-year mortality was rather related to the severe comorbidities, reflected by a considerably higher logistic EuroSCORE I in comparison to other studies (26.5% versus 15.1% to 24.7%)9,15,16,24,25. In general, it seems that nowadays, with refined procedures and devices, risk scores – reflecting the comorbidities of the patients – are less relevant for predicting procedural outcomes and are rather relevant in predicting midterm outcomes22,23. This assumption is confirmed by a recent single-centre study that implanted the ACURATE neo in patients with a substantially lower EuroSCORE I (15.1% compared to 26.5% in our series), reporting a one-year mortality of only 5.2%26.
Despite a rather high post-dilatation rate of 48.3% related to the relatively low radial force of the frame of the prosthesis, only two major strokes occurred. Considering that high-intensity transient signals are mainly observed during valve positioning and implantation27, and first-in-human series generally require more manipulation contributing to the higher risk for cerebrovascular events in the first half of centres’ experiences28, this outcome is encouraging.
The pacemaker rate of 10.3% at 30 days is consistent with those observed for the ACURATE TA prosthesis used for transapical access which ranged between 7.5% and 10.2%4,5. It compares well to those observed for first-generation devices (3.4-50.0%, pooled estimate 13.9%)18, the LOTUS prosthesis (28.6%)17, and the CoreValve Evolut R, Direct Flow, Portico and SAPIEN 3 prostheses which range from 9.5% to 17.1%9,15,16,20,24,25. Of note, the values mentioned refer to the overall study population, whereas the 10.3% event rate in our series refers to patients without a prior pacemaker (using the same calculation, our pacemaker rate would be 9.0%).
The large effective orifice area of 1.77±0.32 cm2 and the single-digit mean gradient reflect the true supra-annular design of the valve. With the caveat that echocardiographic parameters are site reported, only four patients (4.9%) had moderate paravalvular regurgitation and none severe, which is lower than observed for the first-generation devices which ranged from 0% to 30% with a pooled estimate of 7.4%1,2,21, and comparable to recent reports of next-generation prostheses (1% to 3.8%)9,15-17,20,24,25. Echocardiographic outcomes were sustained at one year.
Limitations
This CE-approval cohort has several limitations. The low patient number and lack of randomisation hamper comparisons to other devices. Echocardiographic data are site reported with no echocardiographic core laboratory being used. As enrolment started in January 2012, only VARC-1 criteria were applied8. The strength of the present study is that all patients returned for follow-up assessment. Data on a larger patient number, long-term data and ultimately randomised controlled data comparing different transcatheter heart valves will be necessary to provide further evidence of the usefulness of this device.
Conclusions
In this first-in-human experience in high-risk elderly patients with severe aortic stenosis, the novel ACURATE neo transcatheter heart valve system has shown a low rate of procedural mortality, major stroke and pacemaker implantation, and good performance outcomes. Specific design features contributing to an “ease of implantation” might have positively affected the outcomes in this early series.
| Impact on daily practice We report the first one-year multicentre outcomes of the second-generation self-expanding ACURATE neo transcatheter heart valve. Specific design features allow an easy and intuitive implantation of the prosthesis. In this early series, transfemoral TAVI with the ACURATE neo resulted in good safety and performance outcomes, reflecting the ease of use of the device. |
Acknowledgements
We thank W.D. Weaver, A.P. Kappetein, F.W.A. Verheught, J.G.P. Tijssen, S. Bailey and M. Gorman for their participation in the data monitoring committee, Beatrix Doerr, consultant medical writer, for editorial assistance, and Samuel Copt, consultant statistician, for statistical analysis.
Funding
This work was funded by Symetis SA, Ecublens, Switzerland.
Conflict of interest statement
H. Möllmann and W.K. Kim are proctors for Symetis and St. Jude Medical. D. Siqueira is a proctor for Symetis. P. Diemert is a proctor for Symetis and receives lecture fees. H. Treede is a proctor and advisor for Symetis. T. Rudolph is a proctor for Symetis and has received speaker’s honoraria. J. Kempfert is proctor for Symetis, Edwards and Medtronic. The other authors have no conflicts of interest to declare.

