
Abstract
Aims: The aim of the SAVI TF registry was to assess the safety and performance of the self-expanding ACURATE neo transfemoral transcatheter heart valve in a large patient population with severe aortic stenosis and to investigate whether the outcomes obtained in the CE-mark cohort can be replicated in an unselected all-comers population.
Methods and results: From October 2014 until April 2016, 1,000 patients were enrolled in this prospective, European multicentre registry. Patients were 81.1±5.2 years and had a logistic EuroSCORE II and STS score of 6.6±7.5% and 6.0±5.6%, respectively. Predilatation was performed in 96.1% of patients and post-dilatation in 44.8%. Procedural and device success were both obtained in 98.7%; failure comprised nine valve-in-valve procedures, three conversions to surgery, and one aborted procedure. The primary endpoint was 30-day mortality, which was observed in 14 patients (1.4% [95% CI: 0.7-2.1]). Disabling stroke was seen in 1.2% (95% CI: 0.5-1.9) and new pacemaker implantation in 8.3% (95% CI: 6.6-10.0). At discharge, mean effective orifice area was 1.77±0.46 cm² and mean gradient 8.4±4.0 mmHg; 4.1% of patients had a more than mild paravalvular leak.
Conclusions: In this initial experience, treatment with the ACURATE neo prosthesis resulted in good clinical outcomes with very low complication rates.
Abbreviations
EOA: effective orifice area
NYHA: New York Heart Association
PPI: permanent pacemaker implantation
PVL: paravalvular leak
SAVI-TF: Symetis ACURATE Neo™ Valve Implantation Using TransFemoral Access: SAVI TF Registry
TAVI: transcatheter aortic valve implantation
Introduction
Severe aortic stenosis is a major cause of morbidity and mortality in the elderly. In patients above 75 years, the prevalence of aortic stenosis is 12.4% and the prevalence of severe aortic stenosis 3.4%1. As surgery was denied to one third of elderly patients2, transcatheter aortic valve implantation (TAVI) was developed to offer a less invasive therapy for high-risk patients. Meanwhile, the technique has matured with decreasing complication rates3.
Prior to CE-mark approval, devices are tested in highly controlled clinical studies examining a limited number of patients. It is therefore important that more patients are followed after CE-mark approval to detect possible rare clinical and safety issues in the real-world scenario. The ACURATE neo™ transcatheter aortic prosthesis (Symetis SA, a Boston Scientific company, Ecublens, Switzerland) is a self-expanding valve for transfemoral access, which was built on the ACURATE TA™ transapical platform4,5. The ACURATE neo transcatheter system for transfemoral use has proven to be safe and effective in the first 89 patients implanted, and received CE-mark approval in 20146. The Symetis ACURATE neo™ Valve Implantation Using TransFemoral Access: SAVI TF Registry (SAVI-TF) now aims to evaluate further the safety and performance under real-world conditions in a large patient cohort with severe aortic stenosis.
Methods
STUDY DESIGN AND POPULATION
SAVI-TF is a European, prospective, single-arm, multicentre all-comers registry including 1,000 patients in whom implantation of an ACURATE neo prosthesis was attempted. Implanting centres and numbers are given in Supplementary Figure 1. Inclusion criteria were: 1) eligibility for transcatheter treatment of severe aortic stenosis with the ACURATE neo aortic bioprosthesis and ACURATE TF™ transfemoral delivery system as per instructions for use (age ≥75 years, severe aortic stenosis, and at high risk of surgical aortic valve replacement, as determined by the Heart Team), 2) signed informed consent/data authorisation form, and 3) willingness to return for follow-up visits. Exclusion criteria were ineligibility for treatment with the ACURATE neo aortic bioprosthesis and ACURATE TF transfemoral delivery system as per instructions for use. All centres were aware that a consecutive series of patients receiving the ACURATE neo valve should be obtained. Valve sizing and treatment were conducted per each centre’s standard of care. Follow-up was scheduled at discharge or seven days±1 day (whichever occurred first), at 30±7 days, and at one year post procedure.
The registry was registered at ClinicalTrials.gov (NCT02306226), conducted according to the Declaration of Helsinki, approved by the local ethics committees, and all patients provided written informed consent. To cover the gap between commercialisation and ethics committee approval, hence to ensure that data starting from the first commercially implanted patients were collected, the study protocol allowed retrospective collection of informed consent.
STUDY DEVICE AND PROCEDURE
The ACURATE neo aortic bioprosthesis (Supplementary Figure 2) and its ACURATE TF transfemoral delivery system have been described previously6,7. The valve is available in three sizes (small, medium and large) to accommodate native aortic annulus diameters from 21 to 27 mm. The self-expanding nitinol frame has porcine pericardial leaflets in supra-annular position, treated with the BioFix™ anti-calcification process, and a pericardial skirt acts as a seal against paravalvular regurgitation. At the time of enrolment, the transfemoral ACURATE TF delivery system had to be used with an 18 Fr or larger introducer sheath.
ENDPOINTS AND DEFINITIONS
The primary endpoint was all-cause mortality at 30 days. Secondary endpoints were defined according to Valve Academic Research Consortium-2 criteria and included stroke (disabling and non-disabling), myocardial infarction, bleeding, acute kidney injury, vascular complication, conduction disturbances and other TAVI-related complications8. Furthermore, two combined endpoints were used: “procedural success”, defined as valve implanted in the intended location, aortic regurgitation
STATISTICAL ANALYSIS
No formal sample size calculation was conducted. The analysis is based on the intention-to-treat population and on data available. For quantitative variables, means and standard deviations (SD) were calculated, and for categorical data absolute and relative frequencies. When appropriate, 95% confidence intervals were calculated. Survival curves were constructed using Kaplan-Meier estimates. In a post hoc analysis, patients with and without post-dilatation and patients who signed informed consent prior to and post procedure were compared. Clinical outcomes between these groups were compared using the log-rank test. Comparisons between baseline and follow-up were performed using the Cochran-Mantel-Haenszel test. Data analysis was performed using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
From October 2014 until April 2016, 1,000 patients were enrolled at 25 sites in Europe. None of the sites enrolled more than 15% of the patients (Supplementary Figure 1). Baseline characteristics are provided in Table 1. Patients were 81.1±5.2 years with logistic EuroSCORE II and STS scores of 6.6±7.5% and 6.0±5.6%, respectively (Table 1). Determined by computed tomography and based on subjective estimate of the observer, 33.1% had asymmetric calcification and 33.3% extreme or severe calcification.
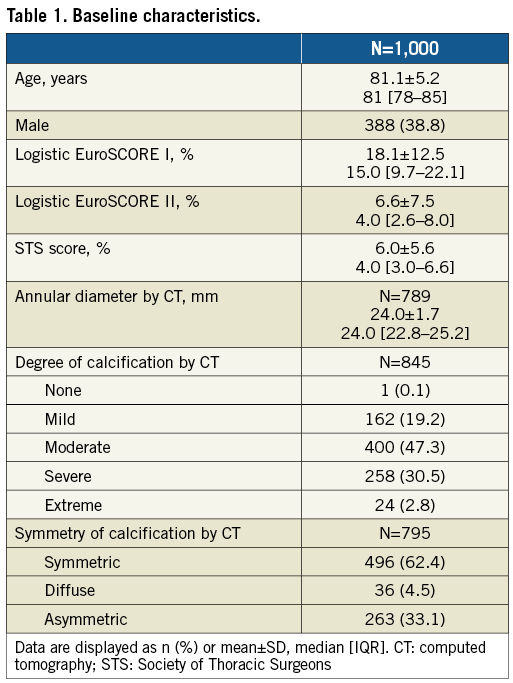
The procedure was conducted under general anaesthesia in 63.1% of patients. In 87.7% of cases, an 18 Fr Cook introducer sheath (Cook Medical Inc., Bloomington, IN, USA) was used. The mean distance to the right and left coronary ostium was 15.0±3.2 mm and 13.3±2.9 mm, respectively. Predilatation was performed in 96.1% of patients, 48.7% of the prostheses were deployed under rapid pacing and 44.8% required post-dilatation. Procedural and device success was obtained in 987 patients each, as valve-in-valve procedures were required in nine patients, conversion to surgery in three patients, and one procedure was aborted (Table 2). By valve size, procedural and device success was 98.9% (n=258), 98.4% (n=424), 99.0% (n=305) for small, medium and large valves, respectively (p=0.717).
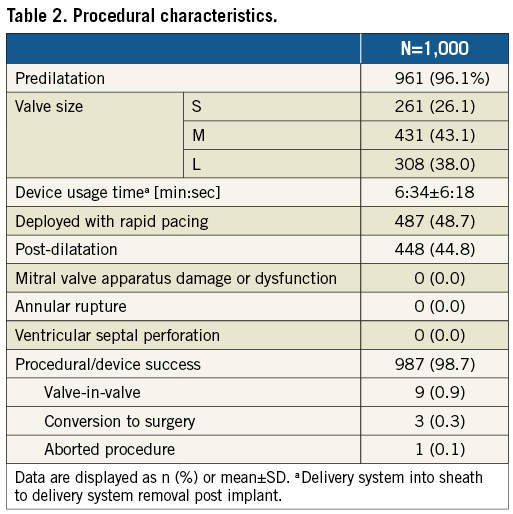
Echocardiographic follow-up at discharge or 7 days±1 day, whichever occurred first, showed an EOA of 1.77±0.46 cm² (95% CI: 1.77-1.85), a mean gradient of 8.4±4.0 mmHg (95% CI: 8.06-8.59), and 4.1% (95% CI: 3.01-5.6) PVL ≥2 (Table 3). By valve size, PVL ≥2 was present in 4.4% (n=10), 3.3% (n=12), and 5.1% (n=13) for small, medium, and large valves, respectively (p=0.409).
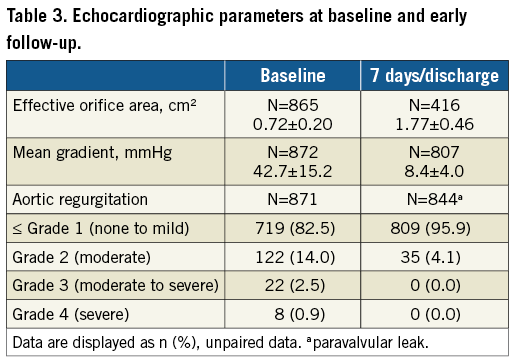
Follow-up data at 30 days were available for 998 (99.8%) patients as one patient withdrew consent and one patient did not return for follow-up. The primary endpoint, all-cause mortality at 30 days, was observed in 1.4% (95% CI: 0.7-2.1) (Figure 1). Comparing outcomes of patients who signed informed consent prior to procedure (n=584) and those who signed informed consent thereafter (n=416), the mortality rate was 1.4% in both groups. The “early safety composite” endpoint occurred in 8.6% (95% CI: 6.9-10.3), disabling stroke in 1.2% (95% CI: 0.5-1.9), and a permanent pacemaker implantation (PPI) was required in 8.3% (95% CI: 6.6-10.0) (Table 4). NYHA class improved significantly (p<0.0001) from baseline with 90.0% of patients in NYHA Class I or II at 30 days (Figure 2). There was no significant difference in clinical outcomes of patients with post-dilatation compared to those without.
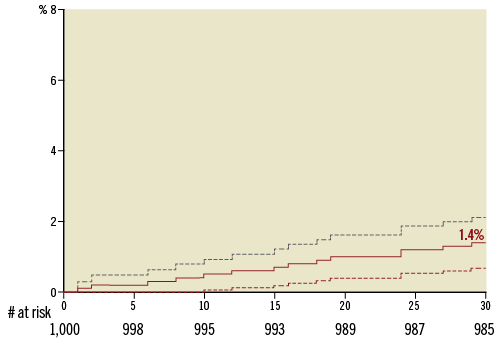
Figure 1. Kaplan-Meier 30-day mortality rate.
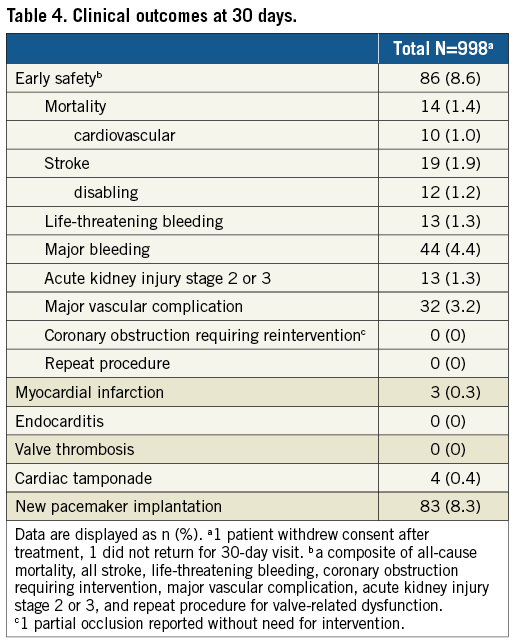
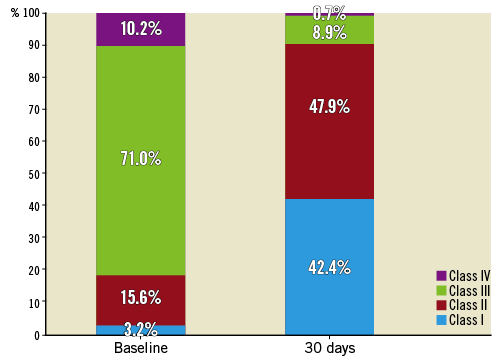
Figure 2. NYHA classification at baseline and 30 days. NYHA: New York Heart Association
Discussion
The main finding of SAVI-TF is that transfemoral implantation of the ACURATE neo prosthesis in an all-comers population under real-world conditions is safe and effective. Complication rates at 30 days were very low; the very low pacemaker rate might make the prosthesis particularly useful for patients with reduced left ventricular ejection fraction5.
The SAVI-TF patient population reflects the common TAVI population with an average age above 80 years and intermediate to high risk scores. No coronary obstruction occurred, and procedure and device success was obtained in nearly all patients (98.7%). The device usage time was short (6:34 minutes from introduction to removal of the delivery system) and was nearly identical to the results of the CE-mark cohort (6 minutes)9.
Clinical outcomes of 1,000 patients enrolled led to small confidence intervals corroborating the reliability of results. Furthermore, results are in line with those obtained in 89 patients of the ACURATE neo CE-mark cohort and in 120 patients of a recently published single-centre experience. In particular, the rate for mortality was 1.4% compared to 3.4% and 2.5%, the rate for disabling stroke was 1.2% compared to 2.2% and 2.7% (the latter displaying the overall stroke rate), and the rate for PPI was 8.3% compared to 9.0% and 9.6%, respectively9,10.
Very good clinical outcomes are also seen in other contemporary TAVI registries. Specifically, at 30 days, the all-cause mortality rates were 3.2% in 317 patients implanted with the CoreValve® Evolut™ R (Medtronic, Minneapolis, MN, USA) in the Swiss TAVI registry11, 2.0% in 250 patients of the Direct Flow registry12, 1.9% in 1,695 transfemoral patients of the SOURCE 3 registry13, 2.6% in 1,014 patients of the Lotus RESPOND registry14, and 2.9-3.6% in 102 patients of the Portico multicentre trial15 and 222 patients of the Portico TF EU study16 as compared to 1.4% in our series. The respective rates for disabling stroke were 1.9%11, 2.0%12, 0.5%13, 2.2%14, and 2.9-3.2%15,16, as compared to 1.2% in our series. Only 3.2% major vascular complications occurred, even though at the time of enrolment the ACURATE TF delivery system had to be used with an 18 Fr or larger introducer sheath, while nowadays it can also be used with a 13 Fr Solopath® system (Terumo Corp., Tokyo, Japan).
The rates for PPI were 22.1% for CoreValve Evolut R, 12.0% for Direct Flow, 12.3% for SOURCE 3, 30.0% for Lotus, and 9.8-13.5% for Portico11-16 as compared to 8.3% in our series. In a recent small study using the ACURATE neo, there were even fewer PPIs required (2.3%). In that study, to minimise trauma, predilatation balloon diameters were selected to be 1 to 3 mm smaller than the perimeter-derived annulus diameter, prosthesis size was carefully selected (size S for annular perimeters <72 mm, M for 72-78 mm, and L for perimeters of 79-84 mm), and post-dilatation balloon diameters were selected to be 1 to 2 mm smaller than the perimeter-derived annulus diameter17.
The supra-annular design of the ACURATE neo led to large EOAs (1.77±0.46 mm2) and low mean gradients (8.4±4.0 mmHg), despite the fact that the valve only accommodates an annular diameter up to 27 mm. The results are similar to those obtained in contemporary registries, such as the ones described above, with the exception of the Direct Flow registry, which reported an EOA of only 1.5 mm² and a mean gradient of 15.8 mmHg12. Further, the LOTUS™ valve (Boston Scientific, Marlborough, MA, USA) showed very low rates of moderate or severe PVL (0.3%) in the RESPOND registry, but at the cost of a high pacemaker rate14. With the ACURATE neo, we observed moderate or severe regurgitation in 4.1%, which is within the ranges observed for the CoreValve® Evolut™ R (8.5%), Direct Flow® (Direct Flow Medical, Santa Rosa, CA, USA) (3%), SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA) (3.1%), and the Portico™ prosthesis (St. Jude Medical, St. Paul, MN, USA) (3.8%-5.7%)11-13,15,16,18. Also encouraging is the functional improvement, with 90% of patients being in NYHA Class I or II at 30 days, reflecting the low invasiveness of the transcatheter procedure with fast recovery.
We did not observe any differences in complication rates between patients with and without post-dilatation. Similarly, post-dilatation of the self-expanding CoreValve prosthesis did not impair clinical outcomes19. In contrast, data obtained from the balloon-expandable SAPIEN prosthesis showed a significant increase of acute and subacute cerebrovascular events in the post-dilatation group20-22. This is of particular importance as major stroke is an important predictor for mortality22. However, it is unclear whether the increased cerebrovascular risk observed with balloon-expandable valves was due to the post-dilatation per se or rather was associated with a high calcium burden, causing a higher likelihood of cerebrovascular events in the post-dilatation group23,24.
Limitations
The limitations of SAVI-TF are those inherent to non-interventional registries with standard-of-care treatment. A major limitation is that informed consent was allowed to be obtained retrospectively, originally to cover the gap between commercialisation and ethics approval. This introduces a bias, as only patients who survived the index procedure were able to provide retrospective informed consent. However, 30-day mortality was the same in the cohort of patients who signed informed consent post procedure compared to those who signed prior to the procedure (1.4%), hence the potential bias is limited. Furthermore, only limited baseline characteristics were assessed, and device and procedural success were not defined according to Valve Academic Research Consortium-2 criteria. There is a lack of echocardiographic data. Additionally, no core laboratories were used, diminishing the validity of echocardiographic parameters and determination of calcification at baseline, the latter being further hampered by the lack of a clear definition. Assessing the severity of paravalvular regurgitation directly after prosthesis deployment and prior to post-dilatation would have been helpful to assess the usefulness of post-dilatation in reducing paravalvular regurgitation and to identify anatomies which might not be responsive to post-dilatation. The echocardiogram was conducted at discharge or seven days. For better comparability, a 30-day follow-up echocardiogram would have been more suitable as –considering the self-expanding nature of the prosthesis– paravalvular regurgitation might have been reduced even further at 30 days compared to discharge.
Conclusions
This European, multicentre, all-comers registry included 1,000 patients and confirmed the very good outcomes of the ACURATE neo CE-mark cohort. Complication rates were very low with –aside from one multicentre study using the same device– the lowest 30-day pacemaker rate for self-expanding transcatheter heart valves published to date.
| Impact on daily practice This is the largest series of the ACURATE neo prosthesis to date. It shows that the prosthesis can be implanted with high procedural success and very low complication rates at 30 days (1.4% mortality, 1.2% disabling stroke, and 8.3% permanent pacemaker). Post-dilatation was frequent, but did not impact on clinical or safety outcomes. Due to these findings, there might be a specific value of the ACURATE neo prosthesis for those patients in whom permanent pacemaker implantation should be avoided. |
Acknowledgements
We thank Beatrix Doerr for her editorial assistance, reimbursed by Symetis SA, Ecublens, Switzerland.
Funding
The study was sponsored by Symetis SA, Ecublens, Switzerland.
Conflict of interest statement
H. Möllmann and W-K. Kim are proctors for Symetis and St. Jude Medical. C. Hengstenberg is proctor for and has received speaker’s honoraria from Edwards Lifesciences and Symetis. M. Hilker, U. Schäfer, and T. Rudolph are proctors for Symetis and have received speaker’s honoraria. A. Linke has received speaker’s honoraria from or served as a consultant for Medtronic, St. Jude Medical, Claret Medical Inc., Boston Scientific, Edwards Lifesciences, Symetis and Bard, and owns stock options from Claret Medical Inc. S. Toggweiler serves as a proctor and consultant to Symetis/Boston Scientific, is a consultant to NVT and has received speaker fees from Symetis/Boston Scientific, Edwards Lifesciences and Medtronic. M. Zembala serves as a proctor and consultant to Symetis/Boston Scientific, AtriCure Inc. and Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. Enrolment per centre.
Supplementary Figure 2. ACURATE neo prosthesis.
To read the full content of this article, please download the PDF.

