The ACURATE neo (Boston Scientific) system is a transcatheter aortic valve implantation (TAVI) system with limited durability data1. We studied all consecutive patients who underwent TAVI with the first-generation ACURATE neo aortic valve system at Karolinska University Hospital between October 2015 and December 2018. The study endpoints were haemodynamic valve deterioration (HVD; stage 2-3), bioprosthetic valve failure (BVF; stage 1-3), and patient-prosthesis mismatch (PPM), as defined by the Valve Academic Research Consortium (VARC)-3 criteria2. Stage 2 HVD is defined as a mean transvalvular gradient increase of ≥10 mmHg, resulting in a mean gradient ≥20 mmHg, or an increase of ≥1 grade of intraprosthetic aortic regurgitation (AR) resulting in ≥moderate AR. Stage 3 HVD is defined as an increase in the mean transvalvular gradient of ≥20 mmHg, resulting in a mean gradient ≥30 mmHg, or an increase of ≥2 grades of intraprosthetic AR resulting in ≥moderate-to-severe AR. Stage 1 BVF is defined as any bioprosthetic valve dysfunction (BVD) with new-onset or worsening symptoms or irreversible stage 3 HVD. Stage 2 BVF is defined as BVD that required aortic valve reintervention. Stage 3 BVF is defined as valve-related death. PPM is defined per VARC-3 with the body mass index-adjusted criteria2.
Echocardiographic follow-up was performed until March 2023. The first and last follow-up echocardiogram was analysed at an external core laboratory. In case of aortic valve reintervention, the last echocardiogram before the reintervention was analysed. Clinical follow-up was by outpatient visits and by scrutinising medical records, including echocardiographic reports from all hospitals in the Stockholm region, until March 2023. Ethical approval was obtained from the Swedish Ethical Review Authority (2021-01224).
During the study period, 452 patients underwent TAVI with the ACURATE neo system. The study cohort consisted of 433 patients surviving 6 months after TAVI. The mean age was 81.0±6.6 years, 54% were female, and the mean European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was 5.2±5.1. A total of 8.3% had bicuspid anatomy, and 1.2% were valve-in-valve procedures. All procedures were transfemoral, and postdilatation was used in 47%. The mean aortic valve gradient was 7.6±3.9 mmHg at predischarge, and the mean aortic valve area was 2.1±0.5 cm2. At predischarge, 14% of patients had moderate paravalvular regurgitation, and 0.4% had moderate valvular regurgitation; none were severe. During follow-up, stage 1 BVF (symptomatic failure) occurred in 0.7% (3 patients), and stage 2 BVF (aortic valve reintervention) occurred in 0.7% (3 patients; at 31, 141 and 151 months after the index TAVI) (Figure 1). One of the reintervened patients had endocarditis; there were no other cases of endocarditis. None of the reintervened patients had more than mild aortic regurgitation on their predischarge echocardiogram. There were no instances of stage 3 BVF (valve-related death).
Echocardiographic follow-up that was analysable by the core lab was available in 379 patients at a median of 39 months (range: 1-69 months) of follow-up. We found a 1.1% rate (4 patients) of severe HVD (all reinterventions were in these patients) and a 1.1% rate (4 patients) of moderate HVD. Two patients with moderate HVD did not have BVF. None of the cases of HVD were caused by stenosis. There was one case of severe intraprosthetic AR; the other three cases of severe HVD had a combination of moderate intraprosthetic AR and paravalvular AR. No abnormality could be seen on the leaflets in any of the cases of HVD. The average mean aortic valve gradient was 7.5±3.6 mmHg post-procedure and 5.7±3.1 mmHg at midterm follow-up (p<0.001; paired t-test). Moderate grade PPM was diagnosed in 6.6% of patients and severe grade PPM in 0.7%. In the 54 patients lacking core lab-analysed echocardiographic follow-up and in the 19 patients who died within 6 months, clinical follow-up revealed no cases of stage 1-3 BVF or any findings suggesting HVD.
Our results are comparable with the 3-year data in the SCOPE I study1, which showed a 0.4% rate of moderate-severe HVD in 251 patients, thus a smaller echocardiographic follow-up group compared to our study. Another study with longer follow-up of ACURATE prostheses3 found a 1% rate of HVD in 104 patients at 3-year follow-up. These studies did not use an external core laboratory. The failure mode was new transvalvular regurgitation in all cases in our study. In contrast, with the CoreValve/Evolut R TAVI system (Medtronic), the predominant failure mode was acquired stenosis, but the total percentage of HVD with patients was similar (2.2% at 5 years)4.
We did not analyse serial annual echocardiograms, which limits the possibility of defining the timing of HVD. In some patients, the follow-up echocardiogram was missing, possibly leading to an underestimation of the rate of HVD. The scrutiny of health records in these patients should have ensured that cases of grade 2-3 BVF (mortality or reintervention) were not missed.
Midterm clinical follow-up of 433 patients at 39 months after TAVI with the ACURATE system showed low rates of BVF, and core lab-adjudicated echocardiographies in 379 patients showed low rates of HVD and PPM. Studies with longer follow-up are warranted.
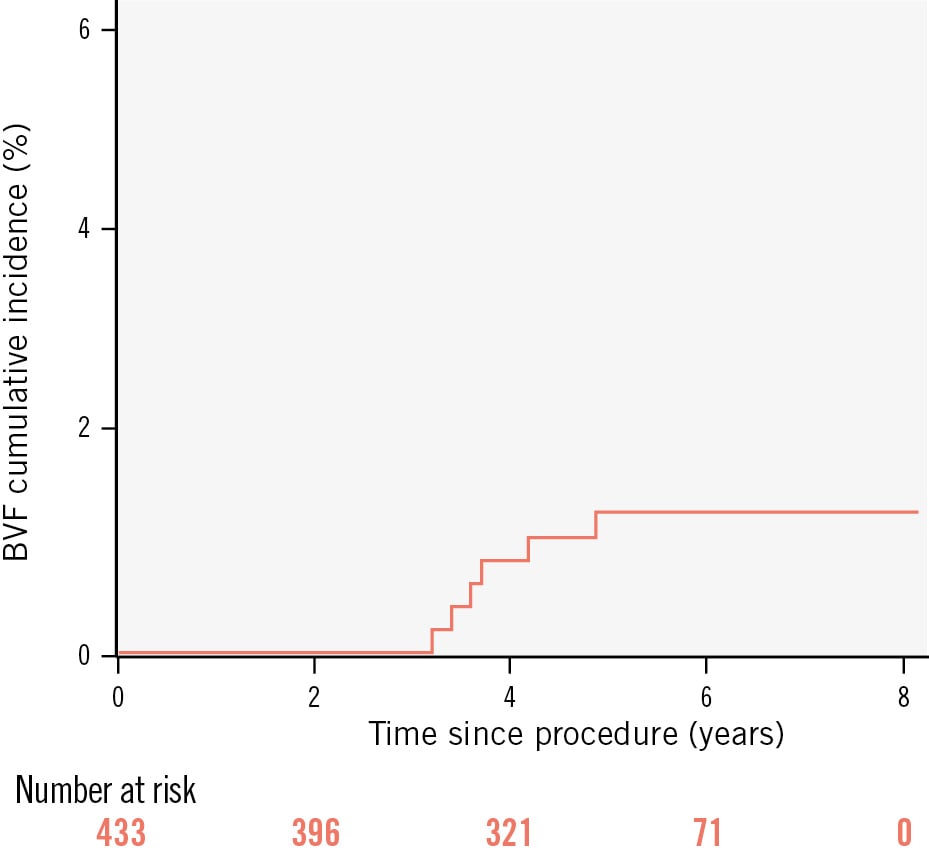
Figure 1. Incidence curve for bioprosthetic valve failure. BVF: bioprosthetic valve failure
Funding
The study was supported by an unrestricted grant from Boston Scientific.
Conflict of interest statement
A. Rück reports grants and personal fees from Boston Scientific; and personal fees from Edwards Lifesciences, Abbott, and Anteris Technologies, outside the submitted work. C. Meduri reports being Chief Medical Officer for Anteris Technologies; and a consultant for Boston Scientific, Abbott, Alleviant, VDyne, Cardiovalve, xDot Medical, and V2V, outside the submitted work. R. Linder reports grants and personal fees from Boston Scientific outside the submitted work. M. Settergren reports grants and personal fees from W.L. Gore & Associates, Abbott, Boston Scientific, Edwards Lifesciences, and Anteris Technologies, outside the submitted work. O. Soliman reports several institutional research grants and service contracts as the chairman of imaging core laboratory for several industry-sponsored trials, for which he received no personal compensation. N. Saleh reports grants and personal fees from Boston Scientific outside the submitted work. The other authors have no conflicts of interest to declare regarding this work.

