Transcatheter aortic valve implantation (TAVI) has been shown to be non-inferior or superior to surgical aortic valve replacement in a series of randomised clinical trials, with evidence dominated by two transcatheter heart valve (THV) landmark devices, namely, the balloon-expandable SAPIEN (Edwards Lifesciences) and the self-expanding CoreValve/Evolut (Medtronic) family of devices. In parallel, there have been prolific efforts to design novel THV prostheses that are increasingly assessed in direct head-to-head THV comparisons. In this context, the ACURATE THV (Boston Scientific) is a supra-annular self-expanding device that provides specific features such as a top-down deployment, a favourable haemodynamic profile with low transprosthetic gradients and a large effective orifice area, low risk of atrioventricular conduction disturbances, provision of commissural alignment and favourable coronary access. Notwithstanding, the first-generation ACURATE neo device failed to demonstrate non-inferiority in clinical outcomes in the randomised SCOPE I and SCOPE II trials as compared with SAPIEN 3 and Evolut R/PRO, related, at least in part, to higher rates of paravalvular leak (PVL)12 (Figure 1). The next-generation ACURATE neo2 THV, incorporating an active sealing skirt, demonstrated a significant reduction in ≥moderate PVL compared with the first-generation ACURATE neo device3. The reduction in significant PVL was sustained when compared with newer iterations of balloon-expandable and self-expanding THVs. In addition, the ACURATE neo2 has demonstrated a low risk of new permanent pacemaker implantation45. As a result, the ACURATE neo2 is emerging as an interesting alternative treatment option for patients undergoing TAVI, with available evidence currently limited to short-term follow-up.
In this issue of EuroIntervention, Kim and colleagues report the 1-year clinical outcomes and device performance of the ACURATE neo2 using data from the ACURATE neo2 Post Market Clinical Follow-up registry, a prospective, multicentre, single-arm, post-market surveillance study6. A total of 250 patients undergoing TAVI with the ACURATE neo2 at 18 European centres (mean age 81 years; Society of Thoracic Surgeons risk score 2.9±2.0%) were included between December 2020 and January 2022. Clinical, echocardiographic, and computed tomographic (CT) follow-up visits at 1 year were completed in 89%, 76%, and 62% of patients, respectively. At 1-year, all-cause death, stroke, and heart failure hospitalisation occurred in 5.1%, 3.0%, and 1.7% of patients, respectively, and a significant improvement in patient health status from baseline was observed (EQ-5D index score: Δ0.037±0.16; p=0.001). The need for aortic valve reintervention was rare (2 patients), and bioprosthetic haemodynamic properties were sustained at 1 year (effective orifice area 1.7±0.4 cm2; mean transvalvular gradient 7.6±3.2 mmHg) with <1% of patients with moderate PVL.
Interestingly, 2 patients experienced clinically relevant valve thrombosis at 1 year. In the SCOPE I trial, patients with the first-generation ACURATE neo THV had a lower rate of valve thrombosis compared with the intra-annular balloon-expandable SAPIEN 3 THV (subhazard ratio 0.16, 95% confidence interval 0.02-1.35) at 3 years, suggesting potential differences in a predisposition to leaflet thrombosis related to valve design2. However, in the current study, four-dimensional (4D)-CT imaging in patients without indication for chronic anticoagulation after TAVI detected some degree of hypoattenuated leaflet thickening (HALT) in one-quarter and one-third of patients at 30 days and 1 year, respectively, with evidence of restricted leaflet motion (RLM) in one leaflet in 13.1% of patients, in two leaflets in 10.5% of patients, and in all three leaflets in 7.2% of patients. Most HALT cases remained unchanged (67%) or worsened (21%) between 30 days and 1 year in the absence of anticoagulation. There was no clinical impact of HALT or RLM on 1-year clinical or haemodynamic outcomes, while long-term follow-up is needed to determine the long-term impact of HALT and RLM on valve durability and clinical outcomes.
The current analysis provides interesting preliminary data on the safety and feasibility of TAVI with the ACURATE neo2 up to 1 year including clinical, functional, echocardiographic and 4D-CT imaging outcomes, setting the stage for more definitive results from the ongoing ACURATE IDE trial (ClinicalTrials.gov: NCT03735667) that directly compares the ACURATE neo2 against the two established landmark devices in a head-to-head randomised comparison.
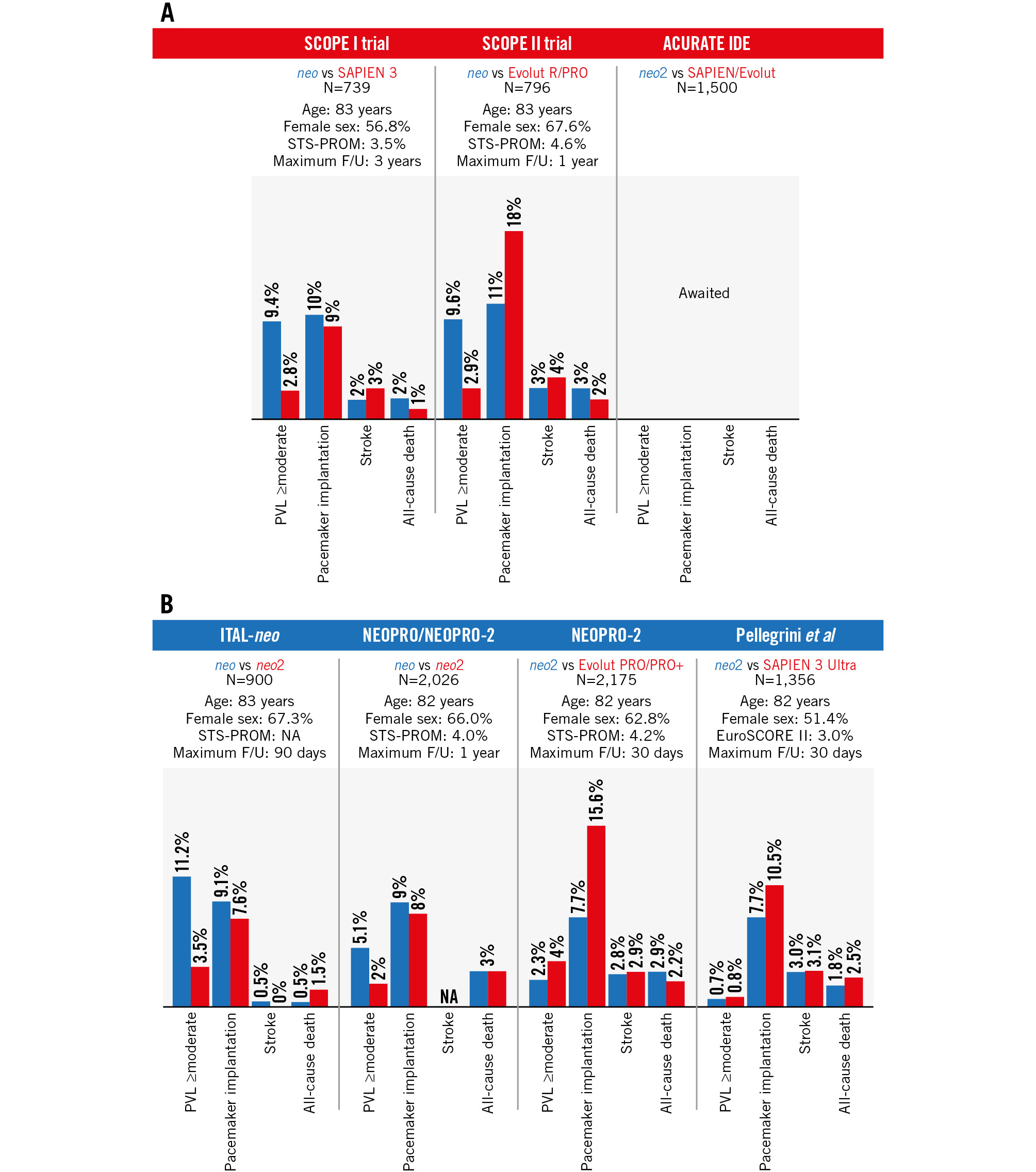
Figure 1. Thirty-day outcomes comparing ACURATE and SAPIEN/Evolut series. Results from (A) randomised control trials and (B) registries. EuroSCORE: European System for Cardiac Operative Risk Evaluation; F/U: follow-up; NA: not applicable; PVL: paravalvular leak; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality
Conflict of interest statement
S. Windecker reports research, travel or educational grants to the institution without personal remuneration from Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Braun, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardinal Health, CardioValve, Cordis Medical, CorFlow Therapeutics, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Farapulse Inc., Fumedica, Guerbet, Idorsia, Inari Medical, Infraredx, Janssen-Cilag, Johnson & Johnson, MedAlliance, Medicure, Medtronic, Merck Sharp & Dohme, Miracor Medical, MonarQ, Novartis, Novo Nordisk, Organon, OrPha Swiss, Pharming Tech, Pfizer, Polares, Regeneron, Sanofi-Aventis, Servier, Sinomed, Terumo, Vifor, and V-Wave; he also served as an advisory board member and/or member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Boston Scientific, Biotronik, Bristol-Myers Squibb, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Recardio, Sinomed, Terumo, and V-Wave, with payments to the institution but no personal payments; and he is also member of the steering/executive committee group of several investigator-initiated trials that receive funding from industry without impact on his personal remuneration. D. Tomii has no conflicts of interest to declare relevant to the contents of this article.

