
Introduction
Transcatheter mitral valve implantation (TMVI) is an emerging treatment for selected patients with severe mitral valve dysfunction that carries an increased risk of left ventricular outflow tract (LVOT) obstruction. Anterior displacement of the anterior mitral leaflet (AML), or bioprosthetic leaflet, by the frame of the implanted transcatheter heart valve (THV) can lead to formation of an obstructed neo-LVOT1,2. Anatomical and procedural factors increase the obstruction risk (Figure 1).
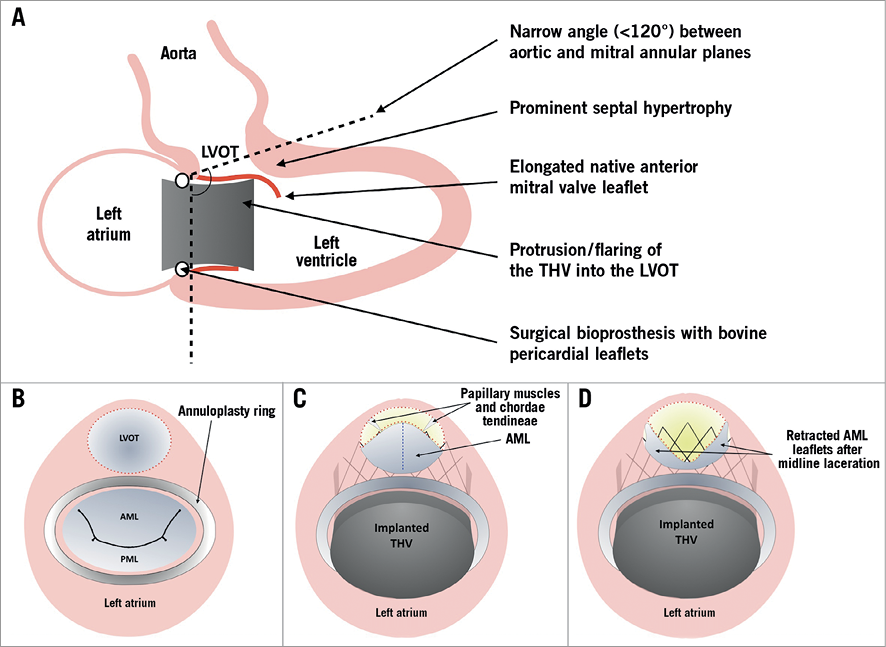
Figure 1. Risk factors for LVOT obstruction during TMVI and the principle of the LAMPOON procedure. A) The primary factors that increase the risk of LVOT obstruction during TMVI. B) - D) The principle of the LAMPOON technique. An illustration of the base of the heart, with the mitral valve and LVOT viewed from above, is shown. Before intervention the LVOT (dotted red line) is widely patent during diastole (B). The struts of the implanted THV cause anterior displacement of the AML, and coverage of the open part of the THV frame, resulting in markedly reduced dimensions of the neo-LVOT (a CT-predicted neo-LVOT area of <189.4 mm² suggests a very high risk of subsequent LVOT obstruction) (C). Midline laceration of the AML from base to tip (dashed blue line) prior to valve implantation can reduce the risk of LVOT obstruction. The leaflets of the lacerated AML are displaced laterally by the papillary muscles, improving LVOT area through the THV frame after deployment (D).
LAMPOON is a novel technique to reduce the risk of LVOT obstruction – in the presence of an intact AML – and facilitate TMVI1,2 (Figure 1). Fewer than 40 cases have been performed worldwide. We describe the first European experience of LAMPOON and discuss the evolution of the procedure.
Methods
An 80-year-old female with multiple comorbidities and symptomatic severe recurrent mitral regurgitation (MR) after previous surgical mitral annuloplasty was referred to our institution. Comorbidities included chronic obstructive pulmonary disease (COPD) with long-term use of supplemental oxygen therapy. Cardiac computed tomography (CT) showed mitral anatomy with a high risk for LVOT obstruction after TMVI due to unfavourable aorto-mitral angulation and septal hypertrophy causing marked visual protrusion of the virtual valve into the LVOT. The patient was deemed inoperable and at prohibitive risk during standard TMVI due to the intact native AML; therefore, compassionate treatment with the novel LAMPOON procedure was recommended.
The routine addition of an arteriovenous rail step has enhanced the original LAMPOON technique. The three key stages of the procedure – 1) arteriovenous rail formation, 2) puncturing the AML and creating a wire loop, and 3) laceration of the AML – are detailed in Figure 2 and illustrated in Moving image 1.
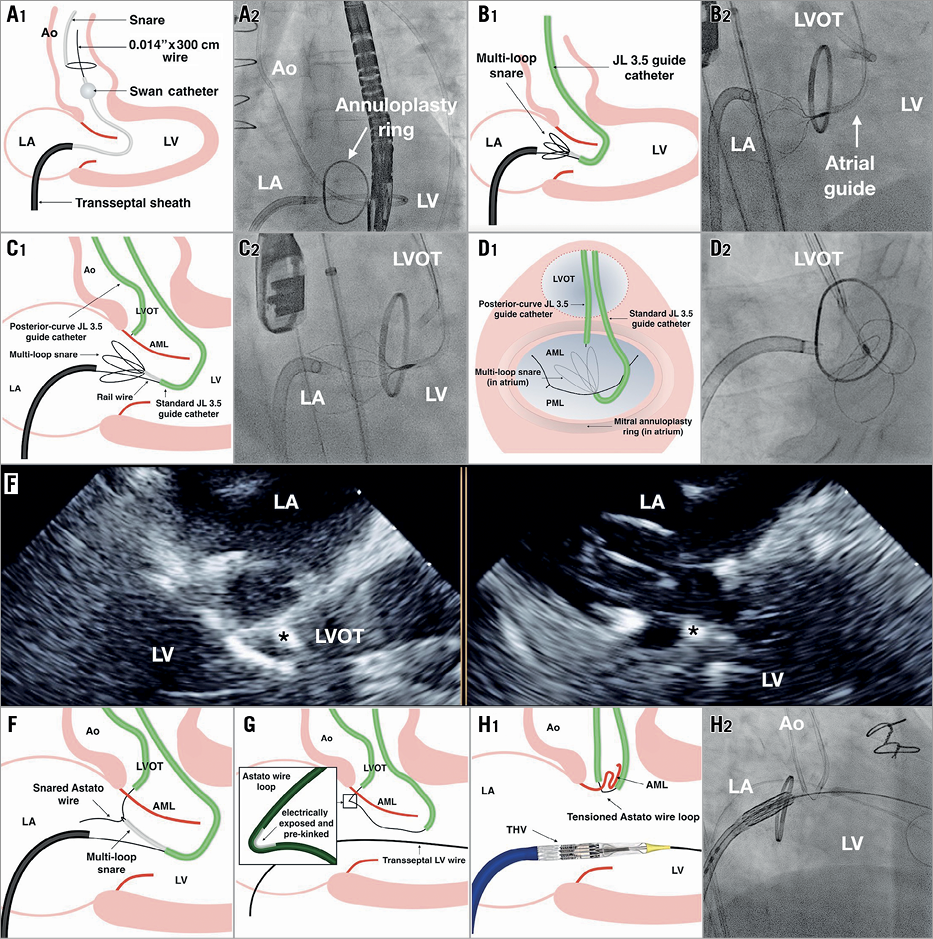
Figure 2. Key angiographic steps during LAMPOON. 1) Arteriovenous rail formation. After transseptal puncture, a Swan-Ganz catheter is floated through the mitral and aortic valves into the aorta, being careful to avoid mitral apparatus entanglement, and a 0.014”×300 cm kink-resistant coronary wire (e.g., Pilot 50; Abbott, Abbott Park, IL, USA) is snared in the aorta and externalised via the femoral artery to create the rail (A1, A2). A multi-loop snare (Atrieve™ 18/30; Argon Medical, Plano, TX, USA) is placed in the left atrium from the arterial side via a 6 Fr Judkins Left 3.5 (atrial) guiding catheter (B1, B2). 2) Puncture of the AML. Puncture of the base of the AML is performed from the ventricular side using a second transarterial 6 Fr posterior-curve Judkins Left 3.5 (LVOT) guide catheter (not currently available in Europe) positioned in the LVOT. An 0.014” x 300 cm traversal wire (Astato XS 20; Asahi Intecc USA, Santa Ana, CA, USA) is locked within an insulating polymer-jacket wire convertor (PiggyBack®; Teleflex Vascular Solutions, Minneapolis, MN, USA); this facilitates later positioning of an electrically exposed pre-kinked segment of the traversal wire. The LVOT guide is aligned in orthogonal angiographic planes – corresponding to three-chamber (C1, C2) and en face (D1, D2) views – towards the base of the A2 segment. Alignment is confirmed echocardiographically (E – guide catheter tip at the base of the AML indicated by the asterisk in the three-chamber [left] and intercommissural [right] transoesophageal echocardiography views). Monopolar electrification (cut mode 50 W) is used to pass the traversal wire through the valve leaflet into the left atrium. The traversal wire is snared (F) and withdrawn via the atrial guide to form a wire loop around the AML from base to tip, with the electrically exposed pre-kinked portion of the traversal wire positioned near the base of the AML (G). 3) Laceration of the AML. The THV is positioned in readiness for prompt deployment and tension is applied to the kinked portion of the AML wire loop via the closely apposed arterial guides. Monopolar electrification is applied to the traversal wire whilst rapidly infusing non-conductive 5% dextrose solution to displace blood and applying further tension to the looped wire (H). Successful laceration of the AML typically occurs within seconds and is indicated angiographically by a sudden release of tension and an upward jump of the arterial guides and wire loop and echocardiographically by new severe MR. After laceration, the THV is implanted in the usual transseptal manner.
Results
The procedure time was 204 minutes, fluoroscopy time 50.3 minutes, and the dose area product 1,556.5 cGy*cm2. An excellent result was achieved with an LVOT mean gradient of 12 mmHg. Our patient remained haemodynamically stable during the procedure, made an uncomplicated recovery, and was discharged on postoperative day three.
Discussion
Together with the support of operators experienced in LAMPOON, procedure planning and equipment selection are key to a successful outcome. Accurate positioning of the posterior curve JL 3.5 guide catheter in the LVOT at the base of the AML during leaflet puncture is challenging as a result of catheter instability and the requirement to confirm angiographic positioning in orthogonal views. Prior to laceration of the AML, care should be taken to avoid excessive tension on the encircling wire loop as this may result in severe mitral regurgitation and haemodynamic compromise.
Limitations
The current complexity of the procedure means that LAMPOON is best performed at centres with high-volume TAVI experience.
Conclusion
Increasing experience with TMVI has led to an improved understanding of the mechanics of LVOT obstruction; however, as a result, a proportion of patients who stand to benefit from TMVI may be declined this treatment due to an excessive risk of LVOT obstruction. LAMPOON represents a novel strategy – supported by promising early safety outcomes – to minimise the risk of LVOT obstruction for selected patients undergoing TMVI.
| Impact on daily practice LAMPOON is a minimally invasive solution for patients otherwise at prohibitive risk of LVOT obstruction during TMVI. |
Conflict of interest statement
M. Kasel and A. Frangieh are proctors and consultants for Edwards Lifesciences. A. Greenbaum serves as a proctor for Edwards Lifesciences. The other authors have no conflicts of interest to declare. The National Institutes of Health and Edwards Lifesciences have a collaborative research agreement on transcatheter modification of the mitral valve.
Supplementary data
Moving image 1. The three key stages of the LAMPOON procedure.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. The three key stages of the LAMPOON procedure.

