
Transcatheter mitral valve replacement (TMVR) is rapidly evolving as an alternative to open surgery for mitral regurgitation and stenosis. For patients with prior surgical valve replacement or repair, valve-in-valve (VIV) or valve-in-ring (VIR) procedures are proven options1. For patients with severe mitral annular calcification, valve-in-MAC (VIMAC) is sometimes feasible2. For patients with native mitral valve regurgitation, approximately a dozen transcatheter valves are currently in human trials. While far from mature, these therapies show promise and will probably become increasingly common.
Early experience with TMVR for failed surgical valves documented the potential for left ventricular outflow tract (LVOT) obstruction. Rarely, transcatheter heart valve (THV) deployment may completely occlude the LVOT, causing immediate haemodynamic compromise and even death. More often, LVOT obstruction is evidenced by a subaortic gradient and suboptimal haemodynamic result. Fortunately, most patients do not experience this complication. Importantly, patients at risk can be reliably identified by appropriate preprocedural imaging.
LVOT obstruction results from a complex interplay between the specific anatomy of the LVOT, left ventricular function, the THV, and either the native anterior mitral valve leaflet or the pre-existing bioprosthetic valve. Echocardiography has been the first line of screening. Small left ventricular size, increased basal septal wall thickness, and a shallow angle (<120°) between the aortic and mitral annular planes all increase the risk. However, computed tomography (CT) cardiac imaging has emerged as the mainstay of preprocedural analysis of the LVOT3. Precise segmentation of the mitral annulus (native, sewing ring or annuloplasty ring) identifies the likely anchoring position and trajectory of the THV. A simulated THV can be virtually placed so as to simulate the potential “neo-LVOT”. The area of this semilunar neo-LVOT is ascertained by planimetric assessment in the best systolic phase. Although the acceptable ranges for neo-LVOT are uncertain, a value of >2 cm2 is associated with low risk of LVOT obstruction4.
Perhaps the most obvious way to avoid LVOT obstruction is open surgery. Surgery allows optimal positioning of the new mitral bioprosthesis, resection of the anterior mitral leaflet, and even myectomy if necessary. The combination of surgical resection of the anterior mitral leaflet with open THV implantation can avoid the need for resection of annular calcium5.
Multiple procedural approaches to reducing the risk of LVOT obstruction are being explored (Figure 1). THVs that are smaller or displaced into the left atrium so as to extend less into the LVOT may reduce risk. Some dedicated TMVR devices for native mitral valve regurgitation are designed to be displaced into the left atrium and subannular (e.g., CardiAQ; Edwards Lifesciences, Irvine, CA, USA), others are asymmetric or D-shaped (e.g., Tendyne [Abbott Vascular, Roseville, MN, USA], Tiara™ [Neovasc Inc., Richmond, BC, Canada]), while others grasp the anterior mitral valve leaflet and withdraw it from the LVOT (e.g., FORTIS™ [Edwards Lifesciences], CardiAQ, Tiara, Caisson [LivaNova, Maple Grove, MN, USA], SAPIEN M3 [Edwards Lifesciences]).
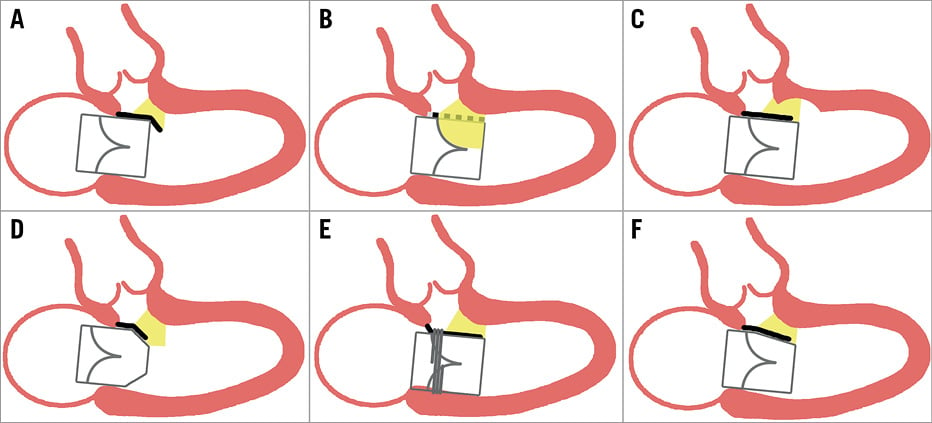
Figure 1. Strategies to reduce the risk of left ventricular outflow tract (LVOT) obstruction during transcatheter mitral valve replacement. A) Subannular (atrial) positioning of the transcatheter heart valve (THV) reduces displacement of the anterior mitral leaflet (AML, black line) into the LVOT. B) Percutaneous splitting (LAMPOON) or removal (surgical) of the AML (broken black line). C) Septal ablation increases the LVOT area and reduces dynamic obstruction. D) A smaller or tapered THV outflow reduces AML displacement. E) Anchors or tabs pull the AML posteriorly. F) Asymmetric or D-shaped THVs increase neo-LVOT area.
In the spirit of “whatever can be accomplished surgically can be accomplished less invasively”, alcohol septal ablation has been performed as a bail-out strategy for severe LVOT obstruction post TMVR6 and, more recently, pre-emptively7. Patients with significant septal hypertrophy and intermediate risk of LVOT obstruction predicted by CT imaging may be best suited. Although anecdotal reports have been favourable, the safety and efficacy of this approach is uncertain.
Similarly, in this issue of EuroIntervention, Kasel et al report the first European experience with Laceration of the Anterior Mitral leaflet to Prevent LVOT ObstructioN (LAMPOON)8.
Briefly, the technique uses transcatheter electrosurgery to transect the A2 scallop of the mitral valve prior to TMVR. Feasibility has been reported in an animal model9 and in limited human subjects10. This builds on increasing experience with electrosurgery in structural heart disease intervention, where the technology has been used for transseptal puncture, transcaval large bore arterial access, pulmonic atresia and aortic coarctation.
With new therapies like TMVR come new concerns. Fortunately, patients at risk of LVOT obstruction can be identified in advance and either avoided or managed with new options such as this.
Conflict of interest statement
J. Webb is a consultant to Edwards Lifesciences and Abbott Vascular. D. Murdoch has no conflicts of interest to declare.

