Pros
David J. Cohen, MD, MSc; Sebastian Ludwig, MD
The emergence of transcatheter mitral valve replacement (TMVR) for the treatment of native mitral regurgitation (MR) was once expected to follow the meteoric trajectory of transcatheter aortic valve replacement (TAVR) by providing simple and safe replacement of the diseased valvular apparatus via a minimally invasive approach. Over the last decade, however, it has become clear that treating the mitral valve represents a much more complex endeavour than TAVR given the saddle-shaped and non-calcified mitral annulus as well as potential interactions with the left ventricular outflow tract (LVOT).
Current issues with TMVR
Early experience with dedicated TMVR devices demonstrated consistent and durable elimination of MR in anatomically suitable patients, with significant symptomatic improvement in survivors at follow-up1. While highlighting the promise of TMVR, these studies have also demonstrated the “Achilles’ heel” of the procedure – unsuitable anatomy with reported screening failure rates of 60-89%234. For example, in the CHOICE-MI registry, the main reasons for anatomical TMVR ineligibility were a high risk of LVOT obstruction, small left ventricular (LV) size, annular dimensions that were outside the range of the available valve prostheses, and mitral annular calcification (MAC)5. The risk of LVOT obstruction posed by devices with large ventricular profiles and covered valve frames is a particular challenge in patients with small LV volumes. Although LVOT obstruction can be modulated using specialised procedures (e.g., LAMPOON or SESAME), these approaches are only partial solutions to this anatomical challenge.
Another key issue for current TMVR devices is the large device profile. Consequently, the majority of TMVR procedures worldwide have been performed via transapical (TA) access. Based on experience with TAVR, however, TA access seems unlikely to provide a meaningful benefit compared with surgery for patients with an acceptable surgical risk. Thus, until transfemoral/transseptal (TF/TS) access for TMVR has been perfected, the need for TA access is likely to limit TMVR to poor surgical candidates.
Current disadvantages of TMVR compared with established therapies
For TMVR to become a viable option for the treatment of patients with severe MR, it will have to provide significant advantages compared with established MR therapies. Given the treatments currently available, this represents a high bar. For repairable valves, surgical mitral valve repair in an experienced centre remains the “gold standard” – offering durable results and sparing the need for long-term anticoagulation. In addition, surgery offers the ability to address multiple issues in a single setting (e.g., other valve disease, coronary artery disease, atrial fibrillation). While these conditions could eventually also be addressed by TMVR with concomitant transcatheter therapies, procedural cost and reimbursement compared with conventional surgery may remain a substantial barrier in many countries.
Among transcatheter therapies, transcatheter edge-to-edge repair (TEER) represents the strongest competitor to TMVR. For many MR patients TEER represents an excellent alternative to surgery by providing substantial MR reduction using a TF/TS approach with minimal procedural risk. Although TMVR offers more effective MR reduction compared with TEER, this benefit comes at a high cost: traumatic large-bore access, elevated procedural risk, and the potential for device thrombosis requiring long-term anticoagulation. Emerging TF-TMVR devices will eventually help to overcome some of these issues and bring the procedural risk closer to that of TEER. But given the current anatomical challenges with TMVR, it is more likely that the role of TMVR in the near future will remain complementary to established therapies – applicable mainly to patients at high or prohibitive surgical risk with TEER-ineligible anatomy or after failed TEER.
Perspectives
Although early TMVR devices continue to evolve, anatomic challenges to widespread adoption of this technology remain substantial (Table 1). In order to compete with established MR therapies, the optimal TMVR device should be suitable for delivery via percutaneous TF/TS access with a low enough profile that LVOT obstruction is minimised. Otherwise, TMVR will most likely remain a niche therapy reserved for poor surgical and TEER candidates.
Table 1. Strengths and limitations of current TMVR devices.
| Strengths | Limitations |
|---|---|
| Predictable and durable MR elimination | Mostly transapical access |
| Treatment irrespective of leaflet/valve anatomy | LVOT obstruction in patients with small ventricles |
| Future valve-in-valve procedure possible | High screening failure rates |
| Risk of device thrombosis | |
| Long-term need for OAC | |
| LVOT: left ventricular outflow tract; MR: mitral regurgitation; OAC: oral anticoagulants; TMVR: transcatheter mitral valve replacement | |
Conflict of interest statement
D.J. Cohen reports research grants and consulting income from Edwards Lifesciences, Abbott, Medtronic, and Boston Scientific. S. Ludwig is supported by a grant from the German Heart Foundation (DHS) and has received travel compensation from Edwards Lifesciences, speaker honoraria from Abbott and advisory fees from Bayer.
Cons
Nicolo Piazza, MD, PhD, FRCPC
The year 2003 marked the world’s first transcatheter mitral valve intervention using the Evalve (Evalve Inc.) – a transcatheter edge-to-edge repair (TEER) technique now referred to as MitraClip (Abbott). Nearly 20 years and 200,000 TEER procedures later, the Carillon Mitral Contour System, a coronary sinus-based indirect annuloplasty device, stands in second place with just over 2,000 implants worldwide since 2008. Here lies the big elephant in the room!
Perhaps the surgical dogma that mitral valve repair is superior to replacement explains the grossly disproportionate number of conceived and studied transcatheter mitral valve repair solutions (e.g., TEER, direct annuloplasty, indirect annuloplasty, chordal implantation) versus replacement solutions. The anatomical, physiological, and pathological complexities of the mitral valve, compounded by imaging and technical challenges, however, have limited the everyday adoption of transcatheter mitral valve repair techniques. Despite its incredible safety profile, the less than perfect ability of TEER to treat mitral regurgitation has operators and patients desiring additional options.
In 2012, Sondergaard et al performed the first-in-human transcatheter transapical mitral valve replacement with the CardiAQ-Edwards Transcatheter Mitral Valve (Edwards Lifesciences). Over the last 10 years, 15-20 different transcatheter mitral valve replacement devices have been implanted in humans (Figure 1). Unlike repair techniques, replacement tends to nearly eliminate mitral regurgitation. Analogous to surgery, transcatheter mitral valve replacement may be more reproducible than transcatheter mitral valve repair procedures. The high number of screen failures related to left ventricular outflow tract obstruction and large mitral annuli, in addition to the engineering challenges associated with lower-profile transseptal delivery systems have hampered widespread adoption. Large transseptal delivery systems, (>30 Fr) explain the higher than anticipated rates of vascular complications and iatrogenic atrial septal defects. Having said that, these engineering challenges do not appear unsurmountable.
Newer designs such as InnovHeart’s Saturn, HighLife’s HighLife, and Edwards Lifesciences’ SAPIEN M3 implement a “valve-in-ring” or docking station that may mitigate the risks of left ventricular outflow tract obstruction and allow the design of lower-profile delivery systems. The “trapping” of the anterior mitral valve leaflet between the prosthesis and docking system may reduce the risks of left ventricular outflow tract obstruction. The 4C Medical AltaValve, an atrial-based solution, completely avoids the issue of left ventricular outflow tract obstruction. Additional innovations in frame and skirt designs (e.g., open-cell concepts) will further reduce screen failures and risks related to left ventricular tract obstruction. The “valve-in-ring” concept avoids the need for radial force and oversizing against the native anatomy and may allow smaller prostheses and, thus, lower-profile delivery catheters for a given annular range. This feature also allows the prosthesis to maintain its nominal shape within the docking station, resulting in greater accuracy and reproducibility of neo-left ventricular outflow tract measurements.
The answer to the question about transcatheter mitral valve repair versus replacement is far from being known and will be multifactorial (Figure 2). Given the clinical equipoise that exists, the ongoing SUMMIT trial is randomising approximately 382 subjects in a 1:1 fashion to the Tendyne transcatheter mitral valve (Abbott) or the MitraClip with a 12-month primary composite endpoint of freedom from all-cause mortality and heart failure.
Suffice to say, we must treat patients as individuals and, therefore, tailor the selection of repair or replacement therapies to the needs of the patient.
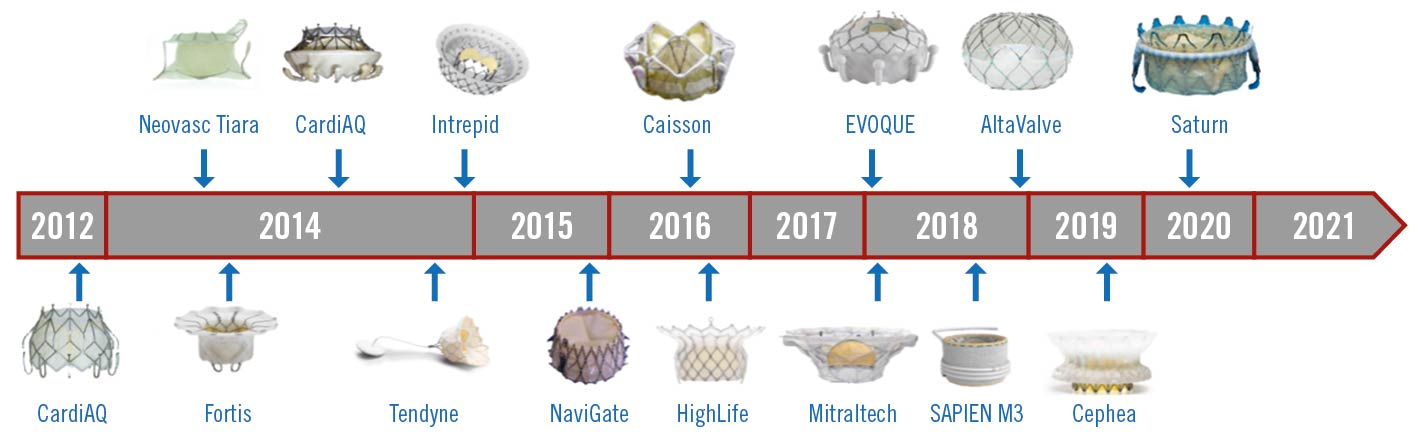
Figure 1. First-in-human timeline for transcatheter mitral valve replacement. Tiara (Neovasc); Caisson (LivaNova); Twelve Intrepid (Medtronic); EVOQUE, Fortis, SAPIEN M3 (all Edwards Lifesciences); AltaValve (4C Medical); Saturn (InnovaHeart); Cephea (Cephea Valve Technologies); NaviGate (NaviGate Cardiac Structures); Mitraltech (Venus Medtech).
Figure 2. Factors influencing transcatheter mitral valve (TCMV) repair or replacement.
Conflict of interest statement
N Piazza is a proctor/ consultant for Medtronic, HighLife Medical, and Peijia.

