Abstract
Over the last few years, several surgical procedures to treat mitral regurgitation (MR) in high-risk or inoperable patients have inspired percutaneous devices, including valve repair and valve replacement technologies. As the field of transcatheter mitral valve intervention is rapidly developing, the interventional community is wondering whether valve implantation should become the leading percutaneous mitral valve therapy, and whether the introduction of reliable replacement technology will reduce the clinical value of repair approaches. Since clinical experience with transcatheter mitral valve implantation (TMVI) is at a preliminary stage and all the patients treated with this approach so far are really sick candidates with prohibitive risk, it is difficult to define properly which patients could benefit more from TMVI versus transcatheter mitral valve repair (TMVR). The specific aim of this report is to state few important clinical and pathophysiological considerations in order to clarify when and why a repair strategy should be preferred over replacement.
Introduction
In recent years, several surgical procedures have inspired percutaneous devices to treat mitral regurgitation (MR) in high-risk or inoperable patients, including valve repair and valve replacement technologies. Today, transcatheter repair with the MitraClip system (Abbott Vascular Inc., Menlo Park, CA, USA) is the most advanced technology available for clinical use, with proven safety, efficacy and durability in different clinical settings1-3. Apart from the MitraClip system, different technologies with diversified approaches are under development to improve the transcatheter mitral valve repair (TMVR) therapeutic spectrum, ranging from leaflet and annular repair to left ventricular remodelling. The feasibility of transcatheter mitral valve implantation (TMVI) was initially shown in patients with previous open heart surgery and a degenerated bioprosthesis or with recurrent MR following surgical annuloplasty (valve-in-valve and valve-in-ring procedures)4,5 or in cases of a calcified mitral valve6,7.
TMVI in native valve anatomy has been reported recently in high-risk patients8, mainly with functional aetiology, with different devices which are under preliminary clinical evaluation (Table 1).
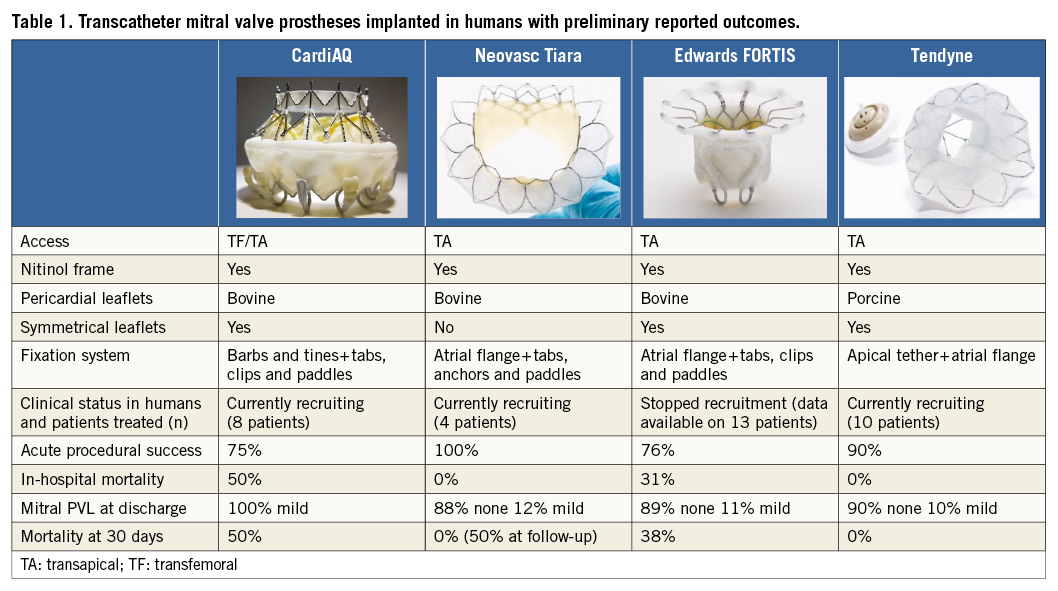
As the field of transcatheter mitral valve intervention is developing rapidly, the interventional community is contemplating whether valve implantation should become the leading percutaneous mitral valve therapy, and whether the introduction of reliable replacement technology will reduce the clinical value of repair approaches.
Since clinical experience with TMVI is really preliminary and all the patients treated with this approach so far are really sick candidates with prohibitive risk, it is really difficult to define properly which patients could benefit more from TMVI than from TMVR. The several technical challenges and unsolved issues that refer specifically to both TMVI and TMVR are not the subject of this manuscript and therefore they will not be discussed here. The specific aim of this report is to state a few important clinical and pathophysiological considerations in order to clarify when and why a repair strategy should be preferred over replacement, in the strong belief that in the near future TMVI and TMVR will play a complementary rather than a competitive role in routine clinical practice.
Valve repair is more respectful of the physiology
The mitral valve is an integral part of the left ventricle (LV). In order to highlight the complexity of the interaction between the valve components and the LV, this anatomical structure is usually defined as the “mitral complex”, composed of the leaflets, the annulus, the chordae, and the papillary muscles. It is also in continuity with the atrial wall and the aortic valve.
From an anatomical standpoint, the mitral valve plays a fundamental role in the demarcation of the inflow-outflow ventricular tracts. This anatomical peculiarity has important haemodynamic implications, since it directs the blood flow through the heart chambers. Imaging studies9,10 have shown that in the LV during diastole the free edge of the anterior mitral leaflet directs the blood flow towards the lateral LV wall. Part of the blood volume is thus redirected towards the outflow tract, according to a vortex-like pattern. Loss of the vortex-like circulation is observed in patients with LV dysfunction and heart failure. This non-physiological flow results in increased LV stress and less efficient work and may contribute to the ongoing maladaptive remodelling process which is observed in heart failure11,12. Since the physiological vortex-like circulation is preserved only after mitral repair, whereas this pattern is compromised after mitral replacement13, it is arguable that a better preservation of the physiology and of the haemodynamics associated with repair could provide improved clinical outcomes compared to valve replacement.
Preservation of the mitral LV structural contiguity
Mitral valve replacement is associated with a discontinuation of the structural contiguity of the mitral apparatus and LV, which results in LV maladaptive remodelling and worse performance, since it affects the reciprocal mitral LV “crosstalk”. Moreover, the fixation of a prosthetic valve to the mitral annulus, which is normally a dynamic three-dimensional structure in direct contiguity to the basal portion of the LV, makes the annulus a static structure. This may be an issue mainly in heart failure patients with reduced EF, since it leads to a reduction of the systolic contribution of the basal portion to the ejection.
Valve replacement is associated with prosthetic-related events
The life expectancy of a patient implanted with a prosthetic valve is reduced, mainly due to thromboembolic and haemorrhagic events and to the risk of prosthetic-related endocarditis14. Thromboembolic complications are the most important cause of morbidity and mortality in patients with a prosthetic heart valve, with an estimated incidence of clinical events ranging from 0.6% to 2.3% per patient-year15,16. The risk of thromboembolic complications is similar for patients with mechanical valves on warfarin therapy and bioprosthetic valves without warfarin therapy. Moreover, obstruction of a prosthetic valve may be caused by thrombus formation, pannus ingrowth, or a combination of these. The incidence of obstructive valve thrombosis varies between 0.3% and 1.3% per patient-year in patients with mechanical valves16,17. Haemorrhagic complications are another major concern related to long-term anticoagulation, with an annual risk of ≈1% per patient-year15,16,18.
The incidence of prosthetic valve endocarditis is ≈0.5% per patient-year, even with appropriate antibiotic prophylaxis. Prosthetic valve endocarditis is an extremely serious condition with high mortality rates (30% to 50%)15,19.
Durability: a limitation of repair and replacement
Durability of a tissue valve in the mitral position is a major concern in surgery. Due to the high-pressure gradient between the left atrium and the LV, the durability of a biological prosthesis in the mitral position is largely suboptimal, especially in patients younger than 65 years. On average, the degeneration process of a biological prosthesis starts five years after operation in the mitral position16. So far, the durability of a transcatheter mitral prosthesis has represented a minor concern, since patients treated are mainly high-risk, elderly or inoperable patients. However, if transcatheter procedures aim to expand indications to a lower-risk and younger population, the durability of the device should be considered a priority.
Durability is of course also a major issue for mitral repair. Acute successful reduction of MR is fundamental in order to provide durable results in TMVR. The four-year results of the EVEREST II trial20 showed that, when the acute procedural result is optimal, transcatheter mitral repair is durable. However, in case of MR persistence or recurrence after mitral repair, outcomes are poor20-24. This suggests that patients eligible for reparative procedures should be treated only in high-volume highly experienced centres.
TMVI and valve thrombosis: need for anticoagulation
Valve thrombosis represents a major issue in this preliminary phase of the development of TMVI. The FORTIS Edwards clinical trial recently stopped patient enrolment to investigate this safety issue further, since evidence of valve thrombosis has been observed in some of the patients implanted. All the patients who undergo TMVI will probably require long-term anticoagulation. Although at the moment no long-term data are available, it is likely that the duration of anticoagulation will be lifelong.
Lessons learned from surgery: the complementary role of repair and replacement in degenerative and functional MR
Although transcatheter interventions may act differently, the surgical background can be a source of inspiration for the future. In patients with degenerative MR (DMR), effective and timely correction of MR has a highly beneficial impact on the prognosis and can even be associated with a life expectancy and a quality of life similar to those of the age-matched general population25,26. The positive prognostic benefit of early intervention in young patients with DMR is lost when a prosthesis is implanted, due to the prosthesis-related morbidity and mortality14.
The benefits of surgical repair over replacement in patients with functional MR (FMR) are less clear compared to DMR patients. Currently, the majority of the patients who undergo transcatheter mitral procedure are high-risk patients with FMR. In surgical practice, mitral repair is more often performed in patients with an earlier diagnosis (smaller ventricle, less advanced anatomical and functional deterioration). In these cases, the chance of successful and durable repair is higher, and prosthesis-related complications are avoided.
Traditionally, mitral valve replacement has been associated with a higher risk of early mortality due to less efficient preservation of left ventricular function. Unfortunately, there is a lack of properly designed studies to support fully the choice between repair and replacement in surgical candidates. Acker et al27 in a large multicentre randomised trial compared mitral repair and mitral replacement in 251 patients with severe ischaemic FMR. The authors did not observe any significant difference in LV reverse remodelling or survival at 12 months, despite a higher recurrence of MR in the repair group. The conclusion was that replacement provided a more durable correction of MR, but this was not associated with a difference in clinical outcome. In heart failure patients with FMR, repair can be beneficial even at the cost of a higher recurrence rate, due to a superior safety profile.
Safety should be the first indicator of success for transcatheter mitral intervention: towards a prognostic approach
Early timing is crucial to achieve a substantial prognostic benefit: restoring expectancy of life in DMR patients and obtaining reverse remodelling in FMR patients21,25. When patients in too advanced a clinical status are treated, outcomes become poor and any intervention is unable to influence the prognosis modifying the natural history of the disease. The impact of any mitral intervention is much more efficient when executed early in the clinical course of the disease. Several issues raise concerns about the role of TMVI in patients in the early stage of their disease. Only a very safe procedure can justify transcatheter therapy as a first-line option. If we consider safety and early indication, repair should be preferred to TMVI due to the lack of the consequences of a prosthesis (including anticoagulant therapy, risk of structural valve deterioration and risk of infection).
Conclusions
From a pathophysiological and clinical standpoint, durability, safety and distortion of the physiology remain major concerns regarding TMVI as compared to TMVR. The impact on physiology is minimal with TMVR and the safety profile is higher. Therefore, in the future, treatment by TMVR may be used following a prognostic approach in lower-risk patients. TMVI will be a complementary therapeutic option for a great number of patients, especially those in an advanced phase of the disease (with both DMR and FMR), who are not amenable to valve repair. Transcatheter mitral repair should, in our opinion, remain the first-line therapy whenever feasible, and should be performed mainly in highly experienced centres. Careful patient selection will be extremely important to define the complementary clinical role of TMVI and TMVR.
Conflict of interest statement
F. Maisano is a consultant for Abbott Vascular, Valtech Cardio, Medtronic, Edwards Lifesciences, St. Jude, and Apica, is co-founder of 4Tech, and receives royalties from Edwards Lifesciences. M. Taramasso has no conflicts of interest to declare.

