Abstract
Abstract: Myocardial infarction with non-obstructive coronary arteries (MINOCA) represents about 6-8% of patients presenting with myocardial infarction (MI), and it is associated with a significant risk of mortality, rehospitalisation, and angina burden, with high associated socioeconomic costs. It is important to note that multiple mechanisms may be responsible for MINOCA. However, to date, there are few prospective clinical trials on MINOCA and the treatment of these patients is still not defined, most likely because of the multiple underlying pathogenic mechanisms. The PROMISE trial is a randomised, multicentre, prospective, superiority, phase IV trial that will include 180 MINOCA patients randomised 1:1 to a "precision-medicine approach", consisting of a comprehensive diagnostic workup and pharmacological treatment specific for the underlying cause, versus a "standard of care” approach, consisting of routine diagnostic workup and standard medical treatment for acute coronary syndrome. The aim of this study is to evaluate if the “precision-medicine approach” will improve the angina status, evaluated using the Seattle Angina Questionnaire summary score, at 12 months (primary endpoint). Secondary endpoints include the rate of major adverse cardiovascular events at 12-month follow-up, the related primary and secondary healthcare costs, and the ability of cardiac magnetic resonance to evaluate the different mechanisms of MINOCA. Of importance, the results derived from this trial may pave the way for a new pathophysiology-driven approach with cause-target therapies personalised for the mechanisms of MINOCA (ClinicalTrials.gov: NCT05122780).
Introduction
Myocardial infarction with non-obstructive coronary arteries (MINOCA) represents about 6-8% of all patients presenting with acute myocardial infarction (MI) referred for coronary angiography (CAG)1. MINOCA is defined by the evidence of MI with normal or near normal coronary arteries in the absence of a specific alternate diagnosis for the clinical presentation (i.e., sepsis, myocarditis, pulmonary embolism and Takotsubo syndrome [TTS]). Notably, there are a variety of causes underlying MINOCA, including coronary plaque rupture (PR)/erosion (PE), epicardial or microvascular spasm, spontaneous coronary artery dissection (SCAD) and coronary microembolism234. Atherosclerotic plaque disruption (including PR and PE) can be identified in up to 40% of MINOCA patients2. The use of intracoronary imaging, and specifically optical coherence tomography (OCT), thanks to its high resolution (up to 10-15 μm), has demonstrated a higher diagnostic accuracy than conventional angiography alone for the detection of PR/PE or SCAD, especially when angiographically non-obstructive lesions are present2. Furthermore, coronary vasomotor disorders (including epicardial or microvascular spasm) are another frequent aetiology of MINOCA which can be elicited by performing intracoronary provocation testing with acetylcholine (ACh) at the time of CAG56. Therefore, MINOCA should not be considered as a single entity but a heterogeneous working diagnosis that requires a comprehensive evaluation to elucidate the potential underlying cause123478. It is important to note that MINOCA is also associated with a significant risk of mortality, rehospitalisation, disability and angina burden, portending high socioeconomic costs9.
To date, however, there are few prospective clinical trials in this population and a treatment algorithm for MINOCA has still not been defined, likely because of the multiple underlying pathogenic mechanisms.
Upon this background, we designed the “PROgnostic Value of Precision Medicine in Patients With Myocardial Infarction and Non-obStructive Coronary artEries” (PROMISE) trial (ClinicalTrials.gov: NCT05122780). Of importance, the PROMISE trial aims to further refine the treatment algorithm for MINOCA patients and to test the prognostic value of a targeted therapeutic approach.
Methods
Study design
The PROMISE study is a randomised, multicentre, prospective, open-label, superiority, phase IV trial comparing a “precision-medicine approach” versus “standard of care approach”, with the aim of improving the prognoses and/or the quality of life of patients presenting with MINOCA. Enrolled patients will be randomised 1:1 to either (1) a “precision-medicine approach”, consisting of a comprehensive diagnostic workup aimed at elucidating the pathophysiological mechanism and consequently a tailored pharmacological approach or (2) a “standard of care” approach, consisting of the standard diagnostic algorithm and therapy for MI. Patients will be enrolled in at least 3 sites in Italy. The respective local ethical committees approved the protocol. The study will be conducted according to the Declaration of Helsinki (1964, and its amendments and clarifications) and EU Directives 2001/20/EC and 2005/28/EC. The International Conference on Harmonisation-Good Clinical Practice will be used as guidance.
Study population, inclusion and exclusion criteria
The study will include 180 patients aged ≥18 years and hospitalised for MINOCA. General inclusion and exclusion criteria are depicted in Table 1. Acute MI will be defined based on the Fourth Universal Definition of MI Criteria (Supplementary Appendix 1)10.
Table 1. Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| – Ability to give informed consent to the study | – Inability or limited capacity to give informed consent to the study |
| – Age ≥18 years– MINOCA diagnosis, defined as:– Acute MI*– Evidence of non-obstructive coronary artery disease on CAG (i.e., no coronary artery stenosis >50%)– No specific alternate diagnosis for the clinical presentation | – Age <18 years– Pregnant and breast-feeding women or patients considering becoming pregnant during the study period– Alternate diagnosis for the clinical presentation– Contraindication to contrast-enhanced CMR (e.g., severe renal dysfunction [glomerular filtration rate <30 mL/min]) or non-CMR-compatible pacemaker/defibrillator– Contraindication to drugs administered: e.g., a history of hypersensitivity to drugs administered or its excipients, significant renal and/or hepatic disease– Patients with comorbidities having an expected survival <1 year will be excluded |
| *definition based on the Fourth Universal Definition of Myocardial Infarction Criteria. CAG: coronary angiography; CMR: cardiac magnetic resonance; MI: myocardial infarction; MINOCA: myocardial infarction with non-obstructive coronary arteries | |
Informed consent
All patients must give informed consent prior to enrolment in the study and before any activity related to experimentation. If the patient is unable to provide written informed consent because of an acute setting, an initial verbal consent from the patient can be obtained. If a patient is providing verbal consent, an impartial witness must be present during the entire informed consent discussion. Where a patient has initially consented verbally, written consent should be sought from the patient as soon as the patient is capable of signing the consent form.
Randomisation
Patients are deemed enrolled into the trial after randomisation and treatment assignment using a web-based randomisation tool, available 24/7 at any of the enrolling sites. After informed consent has been obtained and the index CAG has been performed, revealing non-obstructive CAD, the operator will record the suspected diagnosis at that moment, and patients will be immediately randomised to the “precision-medicine approach” or the “standard of care” approach.
Treatment
Figure 1 depicts the flowchart of the study.
In the “precision-medicine approach” arm, patients will undergo a comprehensive diagnostic workup consisting of:
- CAG and ventriculography
- OCT (suggested in all cases if technically feasible, in particular if coronary PR/PE is suspected) at the time of CAG
- intracoronary ACh provocative test (to assess for the presence of coronary vasospasm) at the time of CAG (performed in all patients, unless clinically contraindicated [i.e., haemodynamic instability, sustained ventricular arrhythmias])
- transoesophageal echocardiography (TOE) and/or contrast-enhanced echocardiography (CEE; if distal/microvascular embolisation is suspected based on the presence of risk factors of thromboembolism, such as atrial fibrillation, mechanical valves, thrombophilic disorders, etc.) during the index hospitalisation
- blood sampling for circulating biomarkers and microRNA (miRNA) expression profile at the time of CAG or within 12 hours
- transthoracic echocardiography (TTE) in all patients during the index hospitalisation
- cardiac magnetic resonance (CMR) in all cases during the index hospitalisation (time frame: from day 3 to day 7 following the acute coronary event)
Further details on diagnostic workup are reported in Supplementary Appendix 3- Supplementary Appendix 7.
Afterwards, a targeted pharmacological treatment specific for the underlying cause will be established (Figure 2), consisting of:
- dual antiplatelet therapy (DAPT) (acetylsalicylic acid and a P2Y12 receptor inhibitor [i.e., clopidogrel])±stent implantation if required (Supplementary Appendix 2), statins, beta blockers, angiotensin-converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARBs) (in case of evidence of PR/PE)
- calcium channel blockers (CCBs) and/or nitrates (in case of documentation of coronary vasospasm)
- anticoagulation (in case of coronary embolism)
Conversely, in the "standard approach" arm, patients will undergo a routine diagnostic workup consisting of:
- CAG and ventriculography without additional diagnostic tests (neither intracoronary imaging nor functional testing)
- TTE in all patients during the index hospitalisation
- CMR only if clinically indicated (e.g., suspected myocarditis or TTS)
Afterwards, a standard medical treatment will be established, consisting of:
- DAPT in all patients
- beta blockers, if clinically indicated (Supplementary Appendix 2)
- high intensity statins in all patients
- ACEi/ARBs, if clinically indicated (Supplementary Appendix 2).
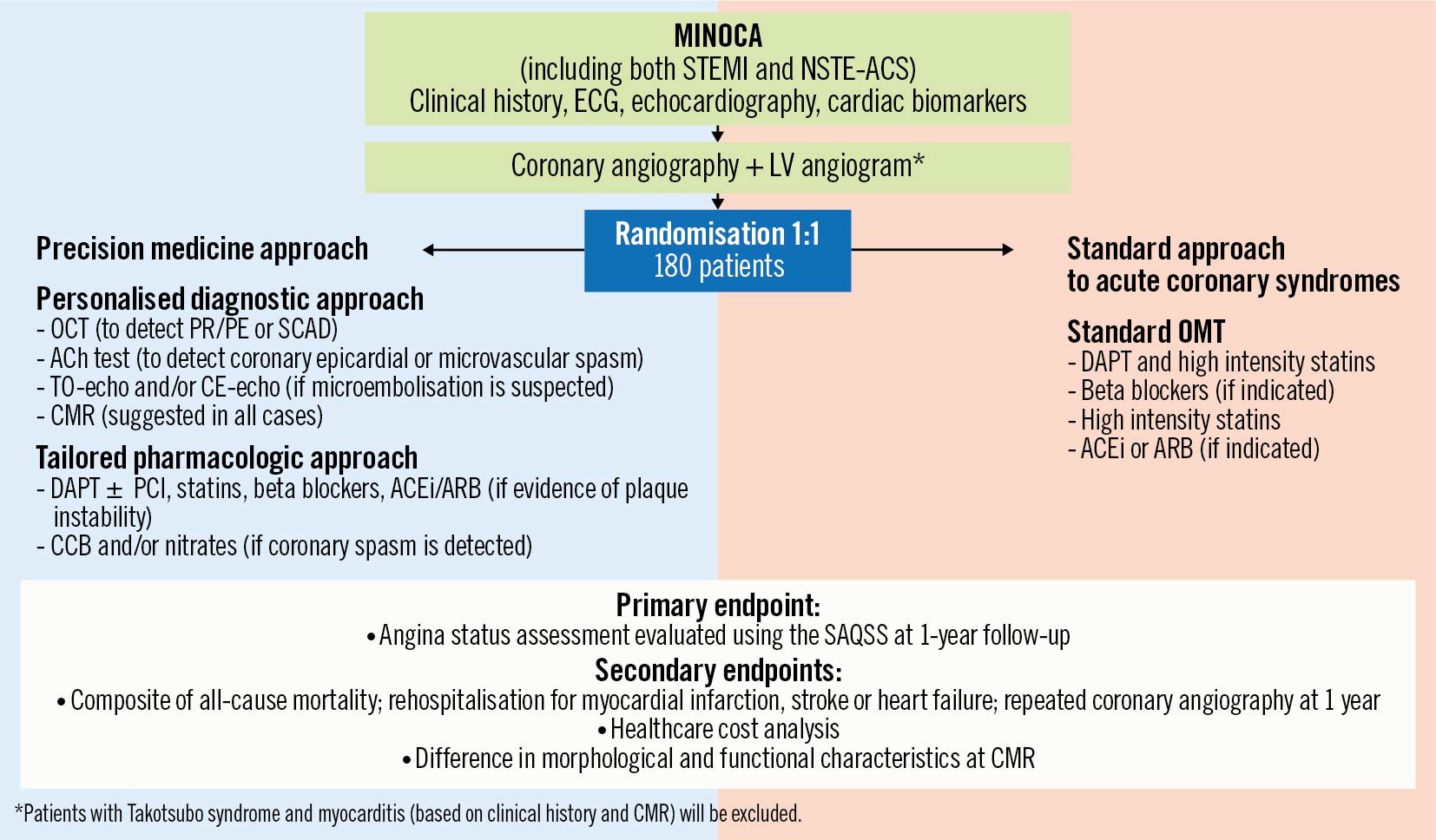
Figure 1. Study flowchart. ACEi: angiotensin-converting enzymes inhibitors; ACh: acetylcholine; ACS: acute coronary syndrome; ARB: angiotensin receptor blockers; CCB: calcium channel blockers; CE: contrast enhanced; CMR: cardiac magnetic resonance; DAPT: dual antiplatelet therapy; ECG: electrocardiogram; LV: left ventricle; MINOCA: myocardial infarction with non-obstructive coronary arteries; NSTE: non-ST-segment elevation; OCT: optical coherence tomography; OMT: optimal medical therapy; PCI: percutaneous coronary intervention; PE: plaque erosion; PR: plaque rupture; SAQSS: Seattle Angina Questionnaire summary score; SCAD: spontaneous coronary artery dissection; STEMI: ST-segment elevation myocardial infarction; TO: transoesophageal.
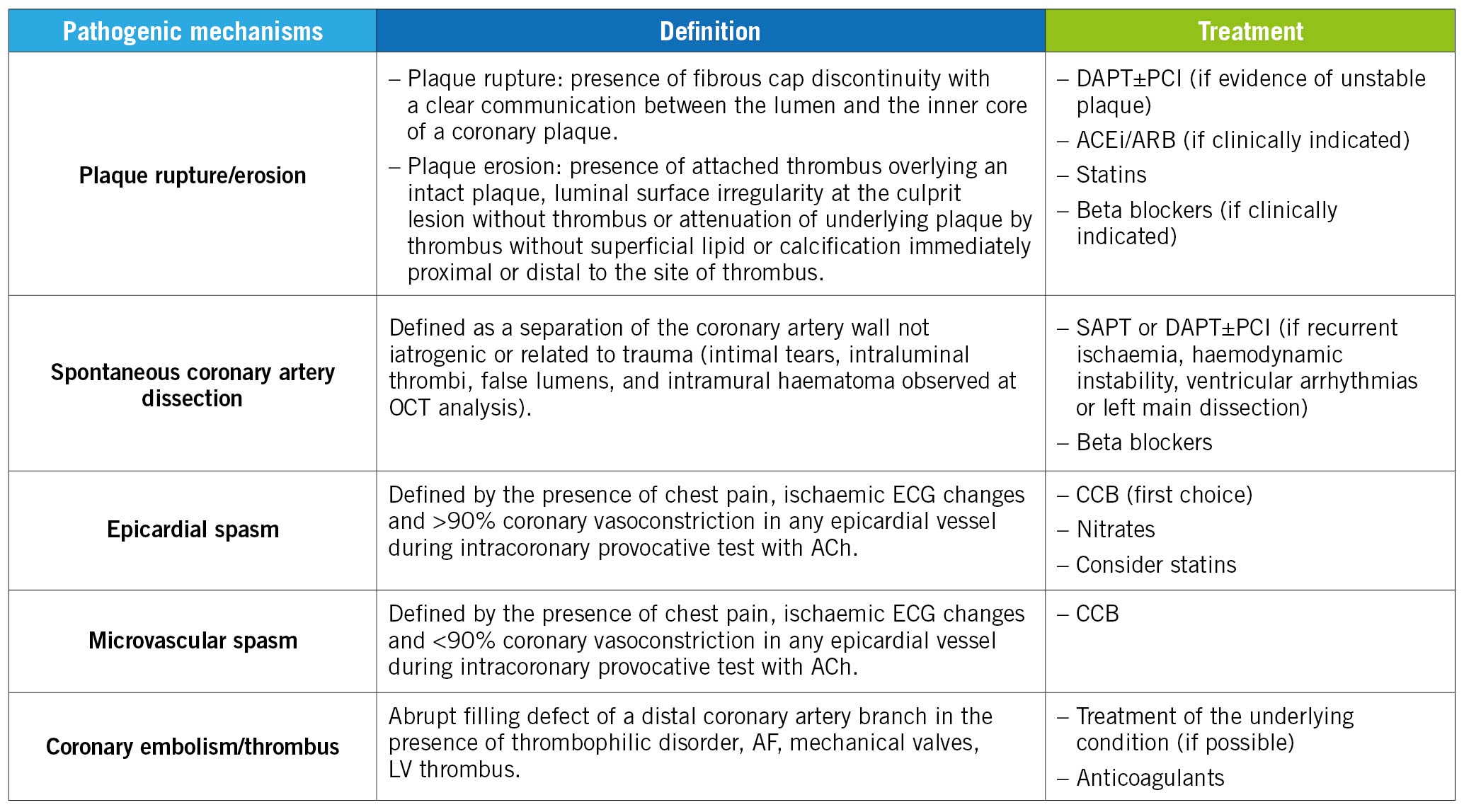
Figure 2. Detailed description of the different pharmacological approaches according to the specific aetiology underlying MINOCA. ACEi: angiotensin-converting enzymes inhibitors; ACh: acetylcholine; AF: atrial fibrillation; ARB: angiotensin receptor blockers; CCB: calcium channel blockers; DAPT: dual antiplatelet therapy; ECG: electrocardiogram; LV: left ventricle; MINOCA: Myocardial Infarction with Non-Obstructive Coronary Arteries; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; SAPT: single antiplatelet therapy.
Follow-up
Patients will be followed up for 12 months after the index procedure. This will include a phone interview at 3 (90±5 days), 6 (180±5 days) and 9 months (270±5 days) as well as a clinical visit at 12 months (365±5 days) aiming to collect vital status, the incidence of rehospitalisation for MI, stroke, heart failure, repeated coronary angiography, the ongoing medical therapy, the occurrence of adverse events and the Seattle Angina Questionnaire (SAQ) summary score (SAQSS)11 at 12 months.
During each follow-up visit, the occurrence of adverse events and serious adverse events (SAE) (e.g., adverse effects of the drugs administered, such as bleeding) will be recorded and monitored (Supplementary Appendix 8-Supplementary Appendix 9).
Endpoints
The primary endpoint is the angina status evaluated using the SAQSS at 12-month follow-up.
The secondary endpoints include:
- rates of major adverse cardiovascular events (MACE; defined as the composite of all-cause mortality, rehospitalisation for MI, stroke, heart failure and repeated CAG) at 12-month follow-up
- related primary (including costs for tests, procedures and outpatient visits or medicines) and secondary (evaluated as mean quality-adjusted life year [QALY] gained and as the incremental cost-effectiveness ratio [ICER] expressed as the cost per QALY) healthcare costs and socioeconomic burden
- the ability of CMR in evaluating the different mechanisms of MINOCA as well as their prognostic value.
Exploratory analysis
The exploratory analysis includes:
- the correlation of different circulating biomarkers (endothelin-1 [ET-1], neuropeptide Y [NPY], C-reactive protein [CRP], soluble CD40-ligand [sCD40L] and miRNA [miR-16, miR-26a, miR-145, miR-222, miR-155-5p, miR-483-5p and miR-451]) with the different pathophysiological mechanisms and clinical outcomes in MINOCA
- in the "precision-medicine approach" arm, both the suspected diagnosis at the time of randomisation (e.g., after diagnostic CAG) and after randomisation (following the additional diagnostic tests) will be collected, to assess the frequency, certainty and change in diagnosis and/or potentially missed diagnosis (diagnostic utility).
In particular, we will assess if miRNA and circulating biomarkers could correlate with different causes of MINOCA, possibly paving the way for future clinical studies to explore their use as diagnostic and stratification tools in clinical practice (Supplementary Appendix 10).
Statistical consideration
In order to detect a mean group difference of change in SAQSS of 9 U, we calculated that a sample size of 70 patients per group (140 patients in total) gave 80% power to detect a between-group difference in SAQSS. This calculation assumed a 2-tailed 5% significance level. This projected calculation assumed a standard deviation (SD) of 19 U and was consistent with previous studies12. However, we extended the sample size to 180 patients to avoid any reduction of statistical power if patients were lost to follow-up or had poor compliance to medical therapy.
Statistical analysis
The analysis will consider the time to first event and time to each event. There will be no interim analyses, and the trial enrolment will be considered complete after the prespecified recruitment target is met. Data will be reported as mean±SD, median (25th, 75th percentile), or frequency and percentage. Continuous outcome measures recorded at baseline and 12 months will be compared between randomised groups using a mixed effects linear regression model, including a random effect for patients, and fixed effects for timepoints (baseline or follow-up), randomised groups and their interaction. The baseline-adjusted intervention effect will be estimated as the interaction term from this model. Categorical outcomes will be compared between randomised groups using Fisher’s exact tests with additional calculation of relative risk estimation of effect size. We will perform a 2-tailed analysis and consider a p-value ≤0.05 to be significant. Statistical analyses will be performed using SPSS (IBM) software.
Discussion
Several studies have demonstrated that MINOCA patients have a 1-year mortality and rehospitalisation rate similar to those patients with acute MI and obstructive coronary artery disease (MICAD)13. Furthermore, approximately 25% of patients with MINOCA will experience angina in the subsequent 12 months, which is at least as high as that reported in patients with MICAD, with a significant impact on quality of life and healthcare related costs14. Angina symptoms also have relevant socioeconomic consequences, as patients with angina without evidence of coronary artery disease (CAD) have the same increased probability of a future disability pension and premature exit from the workforce as patients with obstructive CAD15. Therefore, as a potentially modifiable condition, angina status could be an ideal target to both improve patient symptoms and reduce healthcare costs. This is of crucial importance, because MINOCA patients are usually younger than patients with MICAD. However, less attention has been paid to quality of life outcomes in this population, and whether a pharmacological approach may have an impact on angina burden has never been investigated.
Therefore, a comprehensive evaluation and a multimodal assessment aimed at uncovering the aetiology of MINOCA should be pursued in order to implement a tailored therapeutic approach targeted to the specific underlying cause. Indeed, precision medicine may be particularly important in MINOCA patients because of the multiple causes underlying this condition45678. In addition, such an approach has already proven its effectiveness in patients with stable angina and/or signs of ischaemia with no obstructive coronary artery disease (INOCA) in the landmark coronary microvascular angina (CorMicA) trial, where a strategy of adjunctive invasive testing for disorders of coronary function in these patients linked to stratified medical therapy led to an improvement in patient outcomes, including a reduction in angina severity and better quality of life1216.
Similarly, the identification of functional alterations of coronary circulation is relevant in the clinical setting of MINOCA. Indeed, we previously demonstrated that MINOCA patients with a positive test result (epicardial or microvascular spasm) had a significantly higher occurrence of cardiovascular events, thus identifying a high-risk subset of patients that may need a more aggressive therapy and a closer follow-up1718.
Of note, despite the management of MICAD having well-established evidence-based guidelines, the management of MINOCA still has limited evidence-based literature. Indeed, the current guidelines do not specifically address the issue of acute and long-term management of patients with MINOCA. Moreover, the effects of secondary preventive treatments proven beneficial in patients with MICAD are unknown in MINOCA patients, with few prospective trials exploring this field. In particular, the “Randomized Evaluation of Beta Blocker and ACEI/ARBs treatment of MINOCA patients” (MINOCA-BAT) clinical trial aims to determine whether beta blockers and/or ACEi/ARBs may reduce the composite endpoint of all-cause mortality, readmission for MI, ischaemic stroke or heart failure in MINOCA patients (ClinicalTrials.gov: NCT03686696)19. Similarly, the “Stratified Medicine of Eplerenone in Acute MI/Injury” (StratMed-MINOCA) clinical trial aims to evaluate if a stratified medicine approach, with early risk stratification by coronary microvascular dysfunction (defined as an index of microvascular resistance ≥25), coupled with mineralocorticoid antagonist therapy (i.e., eplerenone) may limit myocardial damage, defined as changes in N-terminal prohormone of brain natriuretic peptide (ClinicalTrials.gov: NCT05198791). Furthermore, the results of a large observational study of MINOCA patients from the SWEDEHEART registry have shown a significantly lower rate of cardiovascular events associated with the use of statins and ACEi/ARBs, and a trend for a lower event rate with the use of beta blockers, while DAPT, a cornerstone of therapy for MICAD patients, failed to improve prognosis. However, an important limitation of this study is the heterogeneous nature of the MINOCA cohort and the difficulty in discerning the mechanism leading to MI2021.
Moreover, information about cardiac structure and function as well as the plasma biochemical profile associated with the different causes of MINOCA is scarce. Beyond its role in differential diagnosis, CMR may help in the morphological and functional cardiac characterisation as well as in the prognostic stratification222324.
Finally, the biochemical profile of patients with MINOCA is still unidentified and whether a unique profile associated with specific pathophysiological mechanisms exists remains unknown. ET-1 and NPY are endogenous vasoactive substances regulating coronary vasomotion, with higher levels circulating in patients with coronary microvascular and epicardial spasm2526. Elevated plasma levels of soluble CD40L and CRP, two inflammatory biomarkers associated with inflammation and thrombus formation, have been described in patients with MI due to acute plaque destabilisation27. Recent findings have shown that levels of specific circulating miRNA may have a close association with specific pathophysiological mechanisms of MI28. Particularly, a unique signature, comprising the upregulation of miR-16 and miR-26a and the downregulation of miR-1 and miR-133, has been reported to characterise coronary microvascular spasm, whereas miR-145 and miR-222 may help to identify patients with epicardial spasm29. Finally, a recent study identified miR-155-5p, miR-483-5p and miR-451a as novel biomarkers for the early identification of PR30. However, the role of circulating plasma biomarkers and miRNA in MINOCA patients has never been investigated.
Limitations
Given the trial design, both physicians and patients cannot be blinded to treatment allocation and, therefore, will know the allocated treatment arm.
Conclusions
In conclusion, the "one-size-fits-all" approach used for MICAD treatment may not apply uniformly to all MINOCA patients, and instead the approach should be "personalised" depending on the underlying pathophysiological mechanism responsible for the clinical presentation.
Thus, the next key step in the management of MINOCA is to demonstrate the benefits of tailored therapies on cardiovascular and quality of life outcomes. With this prospectively designed trial, we will test the prognostic value of a targeted therapeutic approach based on the identification of the underlying cause. Moreover, the results deriving from this trial may pave the way for a new pathophysiology-driven approach with cause-targeted therapies personalised for the mechanisms of MINOCA.
Funding
This study was cofunded by the “Ricerca Finalizzata 2019” Grant (GR-2019-12370197) from the Italian Ministry of Health.
Conflict of interest statement
L. Testa has served as an advisory board member and proctor for Abbott Vascular. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

