Abstract
As a result of the increased use of coronary angiography in acute myocardial infarction in the last two decades, myocardial infarction with non-obstructive coronary arteries (MINOCA) has received growing attention in everyday clinical practice. At the same time, research interest in MINOCA has increased significantly. MINOCA is a heterogeneous disease entity seen in 5-10% of all patients with myocardial infarction, especially in women. Clinically, MINOCA may be difficult to distinguish from other non-ischaemic conditions that can cause similar symptoms and myocardial injury. There is still some confusion around the diagnosis, investigation and management of patients with MINOCA. The present review summarises the current knowledge of MINOCA regarding epidemiology, pathophysiology, investigation, and treatment, with a special focus on imaging modalities. In addition, remaining important knowledge gaps are highlighted.
Introduction
The first paper including the term “myocardial infarction with non-obstructive coronary arteries” (MINOCA) was published in 2013; by the end of 2020, 210 papers had been published. Although it has been known for a long time that myocardial infarction (MI) might occur in the absence of obstructive coronary artery disease (CAD)1, awareness of and interest in the phenomenon among clinicians only gained momentum as coronary angiography became common in the management of acute MI. Although knowledge about MINOCA is rapidly increasing, there are still fundamental gaps in our current knowledge. Furthermore, there is also some confusion and lack of consensus regarding the definition of MINOCA, which is important to bear in mind when interpreting the literature.
DEFINITION OF MINOCA
Myocardial infarction is defined pathologically as myocardial cell death due to prolonged ischaemia. Clinically, elevation of troponin is used as a surrogate for myocardial cell death and, hence, all myocardial injury detected by elevated troponin in the setting of acute myocardial ischaemia should be labelled as MI2. For the diagnosis of MINOCA, the fourth universal definition of myocardial infarction (UDMI) requires that the usual criteria for MI are met and, in addition, no stenosis ≥50% in a major epicardial artery is demonstrated on coronary angiography (i.e., non-obstructive coronary arteries)2. The cut-off of 50% diameter stenosis for defining “obstructive CAD” in MINOCA is based on studies on what degree of stenosis is flow limiting and may cause ischaemia under stress3. MINOCA may be further subdivided into no or very minor atherosclerosis (0-30% stenosis) and minor atherosclerosis with 30-49% stenosis4.
In theory, the diagnosis of MINOCA is clearly separated from MI with obstructive CAD (MI-CAD) as well from myocardial injury of non-ischaemic causes, e.g., Takotsubo syndrome and myocarditis (Central illustration, part A). However, in the real world, due to the lack of specificity of ischaemic symptoms, ischaemic electrocardiogram (ECG) changes and difficulty in measuring the exact degree of stenosis on coronary angiography, the situation is much less clear (Central illustration, part B). Therefore, the European Society of Cardiology (ESC) position paper added a third criterion for the diagnosis of MINOCA: no clinically overt specific cause for the acute presentation other than AMI. Nevertheless, it can be very difficult to exclude other conditions, mainly myocarditis or Takotsubo syndrome, from MINOCA on the basis of the clinical presentation5. Therefore, the initial diagnosis of MINOCA should be considered as a working diagnosis until other causes of the clinical presentation are excluded, preferably by early cardiac magnetic resonance (CMR) imaging4. How to distinguish MINOCA from these two conditions is discussed in more detail below.

Central illustration. Schematic illustration of different conditions that cause myocardial injury, based on whether there is an ischaemic or non-ischaemic cause of the injury. A) In theory, ischaemic and non-ischaemic conditions are clearly separated. B) In clinical practice, the delimitation of the different conditions is sometimes unclear and difficult to determine.
Patients with MINOCA can present themselves with a variation of ECG findings ranging from ST-segment elevation or depression to T-wave inversion or no pathological ECG changes. Hence, MINOCA patients can be classified as ST-segment elevation myocardial infarction (STEMI) or non-ST-segment elevation myocardial infarction (NSTEMI) and meet the type 1 or type 2 MI definitions (Figure 1).

Figure 1. Schematic illustration of the relation between myocardial infarction with non-obstructive coronary arteries (MINOCA) and ST-elevation myocardial infarction (STEMI)/non-ST-elevation myocardial infarction (NSTEMI) and type 1 myocardial infarction (MI)/type 2 MI, respectively.
EPIDEMIOLOGY AND PROGNOSIS
MINOCA is relatively common, a meta-analysis of 28 studies from 1995 to 2013 found that MINOCA accounts for 6% of all MIs6. In sharp contrast to MI-CAD, females are more common than men among MINOCA patients, in most studies >50% are women7. Some, but not all, studies have indicated that MINOCA patients are slightly younger than MI-CAD patients6. Compared to patients with MI-CAD, MINOCA patients have fewer traditional risk factors and previous or concomitant cardiovascular diseases, except for hypertension, which seems to be equally common among MINOCA and MI-CAD patients6,7.
Patients with MINOCA were initially considered to have a benign prognosis. However, larger long-term studies have shown that the prognosis is not benign, with an increased risk of death and new cardiovascular events. A recent meta-analysis of 44 studies showed an annual mortality rate of 2.0%8. Other studies have shown an increased risk of a new MI (in the majority a new MINOCA) and hospitalisation for heart failure and stroke7,9. The maximal level of cardiac troponin during the MINOCA event is associated with long-term mortality10.
DIAGNOSTIC STRATEGY IN MINOCA
The 2019 scientific statement on MINOCA from the American Heart Association introduced a three-step diagnostic strategy in patients with an initial working diagnosis of MINOCA4.
Step 1. Consider the clinical context and exclude different clinical diagnoses, among others, pulmonary embolism and sepsis.
Step 2. Exclude that a true MI-CAD has been missed by re-evaluating the coronary angiogram for missed significant stenosis or side branch occlusion. Exclude other possible non-ischaemic causes of the clinical presentation and the troponin elevation, such as myocarditis and Takotsubo syndrome, by an early CMR examination.
Step 3. Determine the underlying cause of MINOCA, if possible.
If alternative causes for the clinical presentation and myocardial injury are excluded in step 1 and 2, a diagnosis of “true” MINOCA can be made. The second and third steps are described in more detail below.
DIFFERENTIATION OF MINOCA FROM NON-ISCHAEMIC CAUSES OF MYOCARDIAL INJURY IN PATIENTS WITH NO OR NON-OBSTRUCTIVE CAD (STEP 2)
As previously mentioned, sometimes it can be very difficult to exclude other conditions mimicking MINOCA based on the clinical presentation. This is particularly true for Takotsubo syndrome and myocarditis. Typical cases of Takotsubo syndrome can be identified on a left ventricular angiogram and/or an ECG, investigations that should always be performed early in patients with suspected MINOCA. However, for diagnosis of more subtle cases of Takotsubo syndrome or myocarditis, CMR is the method of choice and is recommended routinely in patients with suspected MINOCA4. The prevalence of Takotsubo syndrome and myocarditis in patients with a working diagnosis of MINOCA examined with CMR varies widely in the literature, between 2%-27% and 7%-63%, respectively6,11,12,13,14,15,16. The wide variation is dependent on how the study cohort was selected, the timing of the CMR examination, and the choice of CMR technique and diagnostic criteria.
Late gadolinium enhancement (LGE) on CMR enables assessment of the localisation and size of the infarct in MINOCA. However, in a substantial proportion of patients (8%-73%) fulfilling the diagnostic criteria for MINOCA, and in whom myocarditis or Takotsubo syndrome is ruled out by CMR, it is not possible to demonstrate a localised area of infarction by LGE6,11,12,13,14,15,16. Using contemporary LGE sequences with an average voxel size of 1.4×1.8×6 (–8) mm, at least 0.2 g of infarcted myocardium is required to be detectable by LGE17. The amount of infarcted myocardium required to result in small but significant increases in the concentration of cardiac troponin is much less than 0.2 g18, which may explain why many patients with MINOCA do not show areas of infarction on CMR. However, the possibility that there are causes for the elevation of troponin other than ischaemic necrosis in some of these patients cannot be ruled out.
CMR TECHNIQUES
Standard CMR imaging protocols enable evaluation of cardiac anatomy and function (steady-state free precession [SSFP] magnetic resonance imaging [MRI] or cine images) as well as tissue characteristics. T2-weighted sequences without (T2w) and with fat suppression (T2wSTIR) enable detection of localised oedema, while T1-weighted sequences after gadolinium contrast injection (LGE) permit detection of a localised acute cellular membrane damage and/or a chronic myocardial fibrosis/scar. The extent of LGE allows differentiation between MI and myocarditis with typical subendocardial/transmural (Figure 2A) and subepicardial/mid-wall pattern (Figure 2B), respectively. Typical Takotsubo syndrome presents with an apical and mid-ventricular oedema concomitant with a transient dyskinesia, commonly in the apical and mid-ventricular segments and a hyperkinesia in the basal segments, without any LGE (Figure 2C).

Figure 2. Examples of findings on cardiac magnetic resonance (CMR) and coronary angiography in patients with an initial working diagnosis of MINOCA. A) A 77-year-old female with hypertension, admitted due to chest pain. ECG with T-wave inversions in precordial leads. Hs-Troponin I elevation up to 8,000 ng/L. Coronary angiogram on day 1 showed non-obstructed coronary arteries. Ventriculography revealed a distal apical hypo/dyskinesia, raising suspicion of Takotsubo syndrome. CMR on day 12 showed a normal-sized left ventricle with regional akinesia limited to one apical segment of the anterolateral wall and globally normal systolic function (left ventricular end-diastolic volume [LVEDV] 64 ml/m², ejection fraction [EF] 62%) (steady-state free precession [SSFP] sequence). In the corresponding area, an increased signal in T2wSTIR sequence suggestive of oedema, and a transmural contrast enhancement in LGE sequence, suggestive of a cell membrane damage/scarring, were seen. Final diagnosis was acute myocardial infarction limited to a small area, suggestive of an embolic genesis. The apical segment with respective pathologies indicated by blue arrows. B) A 60-year-old female admitted due to acute chest pain and dizziness, with acute onset during physical activity. Ongoing hormone therapy and radiotherapy after mastectomy two months previously for breast cancer. ECG showed ST-elevations, predominantly in precordial leads. Hs-troponin elevation up to 6,300 ng/L. Coronary angiogram on day 1 showed non-obstructed coronary arteries. Ventriculography was not performed; however, echocardiography revealed an apical dyskinesia suggestive for Takotsubo syndrome. CMR on day 15 showed a normal-sized left ventricle with basal hyperkinesia and apical hypokinesia mainly in the anterolateral wall and a globally normal systolic function (LVEDV 78 ml/m², EF 58%). T2wSTIR sequence showed a clear increased oedema signal in all apical segments. No contrast enhancement was observed in LGE sequences. Final diagnosis was Takotsubo syndrome. C) A 61-year-old male without any cardiovascular risk factors admitted due to a chest pain. ECG showed generalised ST-elevations. Acute coronary angiogram showed non-obstructed coronary arteries. Hs-Troponin I elevation up to >50,000 ng/L. CMR on day 4 revealed a normal-sized left ventricle with regional hypokinesia in mid-apical lateral wall and in mid anterior wall and globally mildly impaired systolic function (LVEDV 89 ml/m², EF 51%) (SSFP sequence). Oedema sequences (T2wSTIR) showed an increased signal in the entire anterolateral wall and in basal-midventricular inferior wall, which also could be confirmed by prolonged native T1-time in T1-mapping sequence. Viability sequences (LGE) show widespread subepicardial and mid-wall signal increase throughout the anterolateral wall, in basal inferior wall, and mid-wall in apical septum. The pattern was suggestive of acute myocarditis.
Some newer MRI techniques can improve diagnostic precision. Use of parametric T1-mapping with calculation of an extracellular volume (ECV), as well as T2-mapping sequences, permits a quantitative pixel-by-pixel determination of tissue features, both extracellular and intracellular, related to the amount of water in myocardial tissue (e.g., inflammation and oedema). It can also identify diffuse changes, such as generalised fibrosis, which LGE cannot visualise. The recently published updated Lake Louise Criteria for CMR in myocardial inflammation introduced mapping sequences and redefined imaging diagnosis according to the combined presence of a T1 criterion (presence of LGE or increased T1-mapping or extracellular volume values) and a T2 criterion (hyperintensity in T2wSTIR or increased T2-mapping values)19. Implementation of mapping techniques has improved the diagnostic performance of CMR for the diagnosis of acute myocarditis, in particular in atypical clinical presentation20.
Recently introduced new free-breathing LGE techniques have substantially improved spatial resolution (up to five times) and permit detection of a smaller amount of necrosis. In a recently published study, by adding this new sequence to the standard imaging protocol, a final diagnosis could be obtained in 48% of MINOCA patients with an initially normal scan21.
TIMING OF CMR EXAMINATION
The diagnostic precision of CMR is increased when imaging is undertaken within 7-14 days of presentation. Delayed imaging may result in certain pathological changes, such as myocardial oedema in myocarditis no longer being apparent. Dastidar et al compared results of retrospective scans in MINOCA patients, showing that early performance of CMR (within two weeks after admission) reduces the number of non-conclusive scans from 43% to 16%, mainly due to a better detectability of Takotsubo syndrome and myocarditis22. In serial scans of patients with a working diagnosis of MINOCA, in whom early scans revealed myocarditis, scans more than three weeks after admission showed a complete resolution of epicardial LGE pattern in one fourth23.
DETERMINATION OF THE PATHOPHYSIOLOGICAL MECHANISMS IN MINOCA (STEP 3)
Coronary angiography is key for the diagnosis of MINOCA. However, coronary angiography has certain limitations for determining its pathophysiology. Angiographically, culprit plaques may seem normal, although there may be findings compatible with MINOCA. These include mild narrowing (less than 50%), lesions with asymmetry, narrow neck, irregular borders, haziness or radiolucent flap5,24,25. Techniques for measuring the functional significance of an epicardial coronary stenosis, such as fractional flow reserve (FFR), are increasingly used. FFR reveals the specific ischaemic potential of the stenosis26. Intracoronary imaging by means of optical coherence tomography (OCT) or intravascular ultrasound (IVUS) offers additional information in terms of the pathophysiology and was therefore recommended in a recent consensus document when diagnostic or angiographic uncertainty exists27.
The underlying pathophysiological mechanisms in MINOCA are still poorly understood. Several different mechanisms for the development of MINOCA have been proposed. Although not mutually exclusive, they can be divided into the following four categories.
1. Processes in the epicardial coronary vessels. This category includes rupture/fissure of small non-significant atherosclerotic plaques, spontaneous coronary artery dissection (SCAD), epicardial vasospasm and in situ thrombosis. Plaque rupture/endothelial erosion are probably quite common causes of MINOCA (Figure 3A); they are dealt with in more detail below. SCAD is an uncommon cause of MINOCA except in younger women28; it is also dealt with in more detail below. Spasm of an epicardial coronary artery may cause transient ischaemia and, in some cases, may even lead to persistent ischaemia, which can cause MINOCA. The diagnostic criteria for coronary spasm have been addressed by the Coronary Vasomotion Disorders International Study Group (COVADIS)29,30. The frequency of epicardial vasospasm as the cause of MINOCA is dependent on the definition of coronary spasm and the cohorts studied. It varies very widely in different studies, ranging from 3% to 95%31,32. Provocative tests for spasm are seldom used in clinical practice in most countries; however, some recent studies have shown the procedure to be safe33. Recently, Reynolds et al identified on OCT a new feature possibly related to vasospasm – superficial bumps34. This finding was reported in 2.1% of patients with MINOCA. In situ thrombosis associated with coagulation disorders and extracardiac embolus are rare causes of MINOCA.
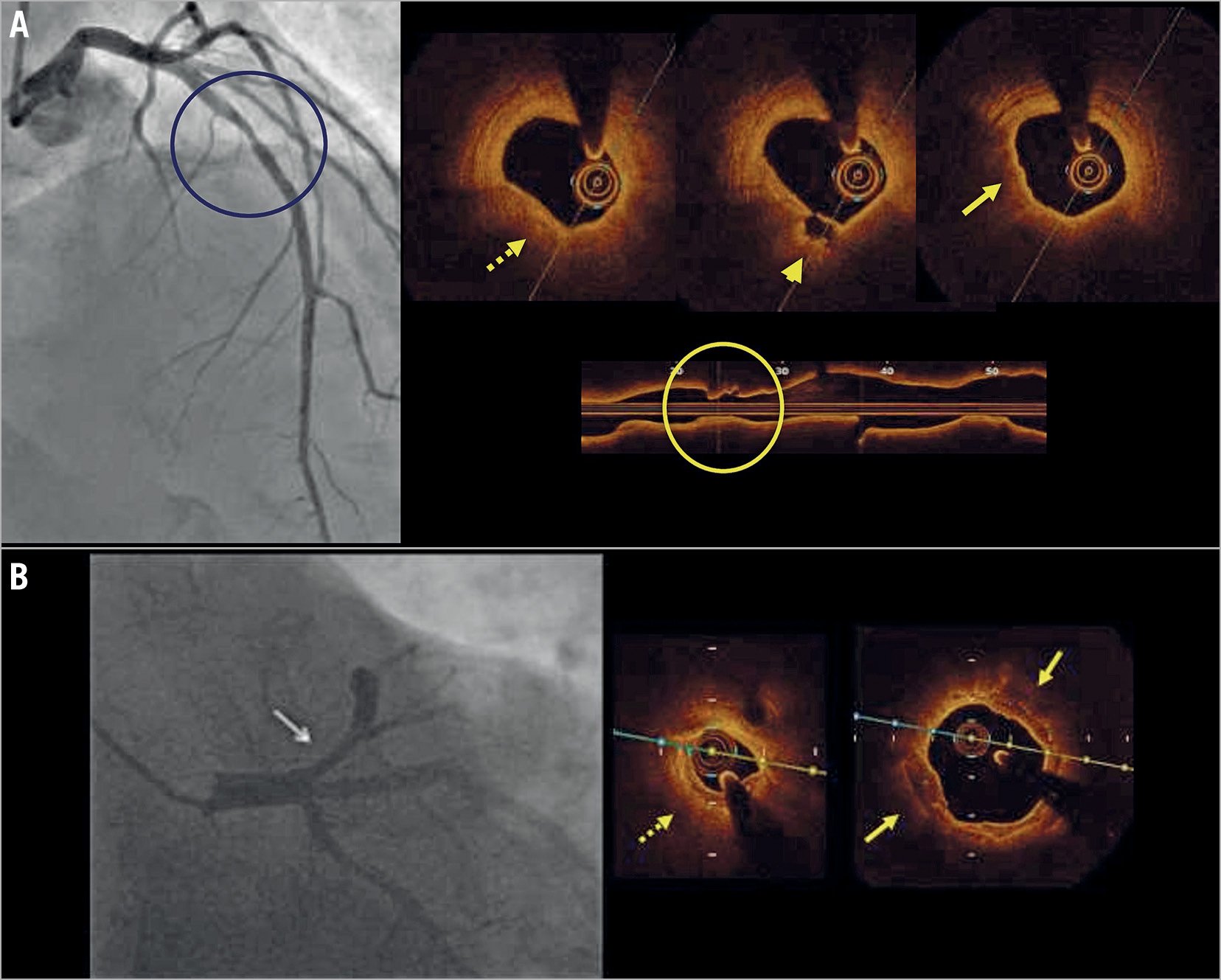
Figure 3. Examples of findings at coronary angiography and OCT imaging in MINOCA. A) A patient admitted with chest pain and anterior ST depression on ECG. Angiography (left panel) shows an intermediate narrowing in the mid left anterior descending artery. OCT showed an atherosclerotic plaque with thin fibrous cap (dotted arrow), a small fibrous cap ulceration (arrowhead) and minimal luminal irregularities due to thrombus remnant (solid arrow). The OCT longitudinal view shows the plaque architecture and plaque ulceration. The findings on OCT confirm the diagnosis of MINOCA. B) A patient admitted to the chest unit for a hypertensive crisis with concomitant anterior ST depression on ECG. Angiography shows an intermediate narrowing in the proximal left anterior descending artery (arrow in the left panel). OCT shows an atherosclerotic plaque with calcific components (solid arrow) in the reference cross sections. At the most narrowed site a small lumen area (less than 3.0 mm²) is present (dotted arrow). The findings on OCT are compatible with a diagnosis of MINOCA due to oxygen supply-demand mismatch (secondary ischaemia).
2. Coronary microvascular disease (CMD). CMD is thought to be a common cause of MINOCA. It is discussed in more detail below.
3. Increased oxygen demand and/or decreased oxygen supply. Increased oxygen demand and/or decreased oxygen supply may lead to myocardial ischaemia and necrosis, i.e., type 2 MI2. Studies have shown that approximately half of the patients with type 2 MI do not have significant CAD, i.e., can be classified as having MINOCA35. The prevalence of type 2 MI is heavily dependent on the studied population. Frequencies of type 2 MI ranging from 2% to 30 % of the total numbers of MI have been reported36. IVUS and OCT can be helpful to define the presence of mild non-significant coronary narrowing that may contribute to secondary ischaemia triggered by arrhythmias, marked anaemia, or respiratory diseases27 (Figure 3B). This mechanism of coronary ischaemia is relatively common in aged people, particularly in the presence of left ventricular hypertrophy.
4. Unknown mechanisms. In a significant proportion of patients with MINOCA the underlying mechanism cannot be firmly established, even after extensive investigations. There are probably still unknown mechanisms behind some cases of MINOCA. Furthermore, in some patients there might be an overlap with mild forms of Takotsubo syndrome, not detectable with current methods.
PLAQUE RUPTURE OR EROSION
MINOCA is caused by atherosclerotic plaque ulceration with superimposed thrombus (plaque ulceration) or by acute thrombosis in the presence of an intact fibrous cap (plaque erosion) in a significant number of cases5. However, MINOCA often shows only small thrombus remnants because of endogenous partial thrombolysis and/or the use of anticoagulant-antiplatelet drugs27. In this regard OCT, with its high resolution in the range of 10-20 microns, is the modality with the most potential27,37. However, some thrombus can obscure the underlying vessel wall imaged with OCT because of its high signal attenuation27,37. The aspect of thrombus on OCT changes over time. The irregular inner border of the thrombus in the acute phase evolves to a homogeneous and smooth profile in the following weeks38. IVUS detection of thrombus is more challenging37,39,40. Stationary imaging at the level of the culprit plaque can be improved by means of a small injection of contrast to highlight the luminal contour. High-definition IVUS has, however, better diagnostic capabilities27.
Plaque rupture is found in about 60-70% of cases of acute coronary syndrome (ACS)2,41 and is also relatively common in MINOCA. Plaque rupture is well recognised by OCT as a discontinuity of the fibrous cap overlying a lipid-rich core that typically occurs with overlying thrombosis. Occasionally, an ulcerated lesion without thrombus may be the cause of MINOCA (Figure 3A). In such cases it may be assumed that local thrombosis responsible for the myocardial injury has been completely cleared or migrated into the periphery. Reynolds et al found layered plaques in 13.1% of patients with MINOCA34. As layered plaques are related to an old plaque healing process38, another underlying mechanism explaining the acute event should be considered in such cases.
IVUS is also able to identify ruptured plaques. However, because of its lower resolution, IVUS provides less clear-cut images of superficial plaque components27. As a main advantage IVUS has better penetration than OCT and can study the outer plaque layers, providing information on lipid content and vessel remodelling37,39,40,42. Near infrared spectroscopy (NIRS)-IVUS imaging provides information about the cholesterol content in the arterial wall, detailing a component of plaque vulnerability43,44. In MINOCA, culprit plaques with ulcerations tend to have large lipid components.
Approximately one third of patients with MINOCA have plaque disruption on IVUS45. Reynolds et al studied 42 women with MINOCA using IVUS, plaque disruption was observed in 16/42 patients (38%)45. Of these, 12 patients had plaque rupture and 4 had only plaque ulceration. These data are consistent with those provided by Hong et al, who performed a three-vessel IVUS study in patients with ACS or stable angina pectoris46. Plaque ruptures were found in 68% and 30% of patients with ACS and stable angina, respectively. Of note, the authors in these studies did not attempt to visualise thrombus with IVUS.
Similar proportions of MINOCA patients with plaque disruption are found in studies using OCT and CMR: Gerbaud et al found plaque rupture in 35%, Opolski et al in 40%, Reynolds et al in 29%, and Taruya et al in 16% of cases12,34,47,48.
Plaque erosion may also be a common cause of MINOCA. Thrombosis without ulceration was found in 30% of cases on OCT by Gerboud et al, and in 50% of cases by Opolski et al12,34,47. However, Reynolds et al found lone thrombus in only 3.5% of cases and Taruya et al found lone thrombus in 8.5% and plaque erosion in 1.2% of cases34,47,48.
Calcified nodules are an infrequent cause of acute thrombosis in ACS (about 5-6% of ACS). They typically occur in aged atherosclerosis and seem unlikely to be involved in MINOCA. In fact, no study has found calcified nodules in MINOCA patients, except Taruya et al, who found calcified nodules in 11.0% of the patients12,34,47,48.
Examination with OCT may also be useful for prognostic purposes. Prati et al found that the simultaneous presence of four high-risk OCT plaque features was associated with a higher risk of major coronary events in patients with a clinical indication to coronary angiography undergoing OCT evaluation of the left anterior descending artery, regardless of the clinical syndrome49.
SPONTANEOUS CORONARY ARTERY DISSECTION
SCAD typically causes ACS. In the majority of cases, SCAD leads to a concomitant significant narrowing (>50% stenosis) and, therefore, is an uncommon cause of MINOCA5. SCAD typically occurs without atherosclerosis and is more common in females50,51. Several conditions or diseases have been related to SCAD, including factors making coronary wall structures more prone to dissection, or factors such as emotional stressors that may precipitate acute episodes of SCAD50. Peripartum is a predisposing condition, making SCAD the main cause of acute MI during pregnancy and the peripartum period52,53. Fibromuscular dysplasia is frequently associated with SCAD, especially when a complete screening is carried out54,55,56. An association also exists with collagen vascular disorders (Marfan syndrome, Ehlers-Danlos syndrome, Alport syndrome and nail-patella syndrome)57,58, chronic inflammatory diseases (systemic lupus erythematosus, inflammatory bowel disease and sarcoidosis)59,60,61,62, and periadventitial eosinophils infiltrate63.
SCAD is characterised by the development of a false lumen within the coronary artery wall that may compress the true lumen and cause ischaemia5,64. Two mechanisms have been proposed to explain the development of the false lumen in SCAD65,66. The first one is the so-called “inside-out” hypothesis, the cause resides in the disruption of the endothelial-intimal layer, which allows blood from the lumen to enter the vessel wall, leading to the formation of an intramural haematoma. According to a second “outside-in” hypothesis, the primary event is a bleeding of the vasa vasorum that leads to intramural haematoma in the absence of intimal disruption. In both scenarios, haematoma can progress and lead to the compression of the true lumen, resulting in myocardial ischaemia66,67. On a positive note, SCADs often heal spontaneously. Observational studies have shown angiographic “healing” of SCAD lesions in a large percentage of cases, between 70% and 97%68,69. Healing tends to occur in an early phase (within days) and is a common finding after the first month50,70.
In general, coronary angiography is the key diagnostic tool50,70. Although angiography does not enable imaging of the arterial wall, it portrays unique disease aspects of SCAD. Based on the angiographic pattern, three types of SCAD can be identified (Figure 4). Type 1 lesions are defined by the presence of a double lumen image, Type 2 lesions by the presence of a long lumen narrowing, usually with a length over 20 mm. Two subtypes of SCAD type 2 have been identified: type 2a, when the distal vessel recovers the normal size; and type 2b, when the intramural haematoma extends distally to the end of the coronary artery. Lastly, type 3 lesions, which are defined by an abrupt focal narrowing (lesion length <20 mm) therefore mimicking an atherosclerotic lesion28,50,51,67. In the SCAD registry by Saw et al, the most commonly observed angiographic appearance was type 2 (67.0% of cases), with type 1 occurring in 29.1% and type 3 in 3.9%50. There were no differences in the baseline characteristics or clinical outcomes according to the angiographic subtypes.

Figure 4. Three types of SCAD. Left panels. Schematic figure of the three different types of dissections. Reprinted with permission from55. Right panel: OCT image illustrating a type 3 dissection with vessel haematoma (asterisks) and the presence of the inner three vessel layer at the site of dissection (dotted arrow).
At present, computed tomography coronary angiography (CTCA) use has important limitations for diagnosing SCAD. In the presence of SCAD, CTCA can reveal an abrupt luminal narrowing, followed by intimal media haematoma identification. Unfortunately, CTCA sensitivity is suboptimal because of its poor spatial resolution; therefore, it does not seem suited for identifying distal or mild lesions71.
Use of other imaging modalities for a correct diagnosis of SCAD is often superfluous. In the presence of type 1 and type 2 SCAD, angiography plus clinical and demographic variables are largely sufficient to make a correct diagnosis. However, both IVUS and OCT are helpful in type 3 dissections that have angiographic features that resemble those of atherosclerotic lesions (Figure 4). OCT may identify the rupture site (the entry tear), visualise the intima-medial membrane and comprehensively assess its characteristics, and the extent and distribution of the true and false lumen/intramural haematoma67. IVUS has two advantages over OCT: it has greater penetration, showing the entire intramural haematoma, and is perhaps safer as it does not require contrast injection. However, this is at the expense of a lower anatomical definition. The visualisation of localised fenestrations, intimal flaps or true luminal thrombus do not seem possible with IVUS.
The benefits of intracoronary imaging modalities should be balanced against the potential complications caused by vessel instrumentation, insertion of intracoronary catheters may lead to propagation of dissection72. Compared to IVUS, OCT has adjunctive limitations. The necessity of contrast injection, might potentially further expand the dissection by hydraulic pressure.
A computed tomography (CT) scan is extremely helpful for identifying extra-coronary vascular abnormalities, including fibromuscular dysplasia. According to Liang et al, comprehensive CT protocol identified fibromuscular dysplasia in 69% of patients with SCAD73. The US Registry for Fibromuscular Dysplasia showed that the most commonly involved vascular beds are the renal (79.7%), followed by the extracranial carotid arteries (74.3%)74,75. Intracranial aneurysm was reported in 14% to 23% of cases50,73.
CORONARY MICROVASCULAR DISEASE
The coronary microcirculation has a fundamental role in the regulation of coronary blood flow in response to cardiac oxygen requirements. Myocardial oxygen extraction is almost maximal at rest in the heart. Therefore, an increase in oxygen demand must be matched by a corresponding increase in coronary blood flow. Coronary microvascular disease (CMD) hampers the necessary increase in coronary flow in response to increased oxygen demand and may lead to myocardial ischaemia.
Nearly one half of patients with stable chest pain undergoing coronary angiography do not show obstructive coronary lesions. A substantial proportion of these patients present abnormalities in the function and structure of the coronary microcirculation due to endothelial and smooth muscle cell dysfunction, defined as CMD. Among consecutive emergency room (ER) chest pain patients without evidence of MI or CAD, 42% had signs of CMD on Rb82 cardiac positron emission tomography (PET)/CT, 36% had CAD and 22% normal flows76. CMD is strongly associated with the development of heart failure with preserved ejection fraction, diabetes, hypertensive heart disease, and carries an increased risk of adverse cardiovascular clinical outcomes77.
Microvascular dysfunction is considered to be a potential cause of MINOCA4, even although not extensively studied as in stable chest pain patients. It is has been estimated that CMD, expressed by coronary microvascular spasm, accounts for about 20% of MINOCA patients5,6. CMD can cause ischaemia, but it can also be a consequence of myocardial injury of either ischaemic or non-ischaemic origin. Using stress CMR, an abnormal stress perfusion was found in 63% women with MINOCA and was associated with myocardial oedema, but the abnormal stress perfusion matched with a myocardial scar in only 75% of cases78. The precise contribution of CMD in MINOCA requires additional studies in order to assess the roles of microvascular angina, microvascular spasm, and the coronary slow flow phenomenon.
Diagnosis of CMD is usually based on functional assessment of the microcirculation, which can be performed by both invasive and non-invasive methods. Invasively, both microvascular vasodilatation (measured by coronary flow reserve and index of microvascular resistance) and microvascular spasm (induced by intracoronary acetylcholine infusion) can be measured. Although being the reference standard for the diagnosis of CMD, the invasive tests of coronary artery function are not widely used in clinical practice, due to their time-consuming complexity and, at many sites, a lack of clinical experience. Non-invasively, myocardial perfusion can be assessed by PET and CMR. PET is the reference standard for non-invasive assessment of myocardial blood flow and coronary flow reserve, calculated as the ratio of myocardial blood flow during pharmacologically induced maximal hyperaemia and at rest79. Its availability, cost and exposure to radiation are limiting factors for a wide use of PET. Stress CMR are also used to assess myocardial perfusion and assessment of microcirculation. The images are acquired at rest and after vasodilator-stress (i.e., adenosine), associated with gadolinium-based contrast agent injection80. The largest study to date comparing CMR imaging and 15O-H2O PET showed that, despite a modest agreement of myocardial blood flow and coronary flow reserve, the concordance regarding vascular territories was satisfactory81.
TREATMENT OF MINOCA
MEDICAL TREATMENT
Evidence-based treatments for MINOCA are lacking since there is no published randomised clinical trial on MINOCA, although there is one ongoing randomised clinical trial82. The treatment recommendations in current guidelines are based mainly on expert opinions. An obvious complicating factor is that MINOCA is a heterogeneous condition and, in clinical routine, the exact underlying mechanism will most often be unknown in the individual case. Furthermore, even if the likely underlying mechanism is known, appropriate treatment remains to be proven. For example, compelling evidence for a beneficial effect of dual antiplatelet therapy (DAPT) in the case of a small plaque rupture in a non-significant stenosis and without overlying thrombus is lacking.
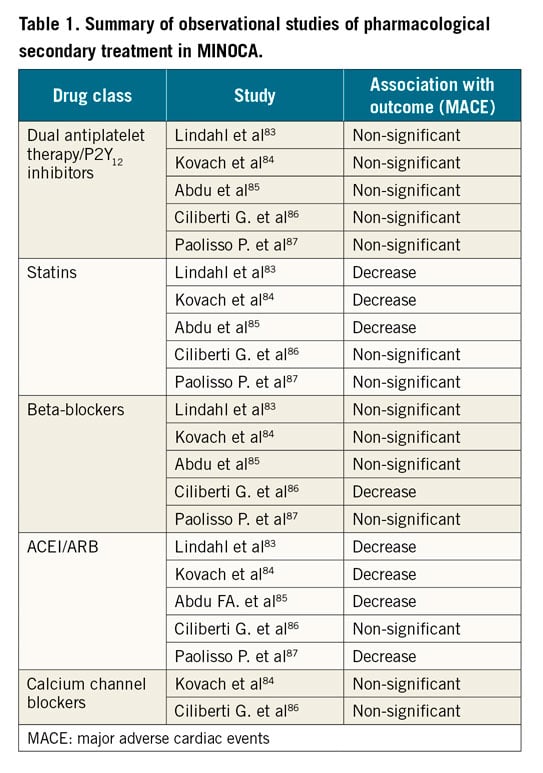
Acute treatment purely provides symptomatic relief and there are no proven infarct size-limiting treatments in MINOCA. Except in SCAD, long-term low-dose aspirin is recommended for secondary prevention after MINOCA in recent consensus documents4. DAPT treatment is controversial, but has not been shown to be associated with lower risk in any observational study so far83,84,85,86,87. A post hoc analysis of the CURRENT-OASIS 7 randomised trial comparing high dose versus standard dose of clopidogrel even indicated possible harm of high-dose clopidogrel in patients with MINOCA as opposed to in patients with MI-CAD88. In contrast, statin treatment has mostly been shown to be associated with lower mortality and a lower rate of MACE83,84,85,86,87, a relative decrease of the same level as was shown in randomised trials of secondary prevention with statins in MI patients89. Similarly, mostly positive results have been shown for angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB)83,84,85,86,87. On the other hand, most studies on beta-blockers have not found significant association with outcome83,84,85,86,87. In the large study of Lindahl et al, there was a non-significant trend for a beneficial effect83. For calcium channel blockers, the data are more scarce, no study has shown an association with a lower long-term risk of MACE84,86. The findings in the observational studies are summarised in Table 1. It should be noted that several of the studies are very small with low power to show significant associations. For non-pharmacological treatments, the data are even scarcer; however, one study has indicated a positive effect of physical training in MINOCA90.
Since a not negligible number of patients experience angina pain after an episode of MINOCA91, symptomatic antianginal treatment with beta-blockers, calcium channel blockers or long-acting nitrates is often needed. If vasospasm is the underlying mechanism, calcium channel blockers are the most effective symptomatic therapy; nitrates represent another option. In case of reduced left-ventricular function, treatments such as ACEI or ARB, beta-blockers and other evidence-based treatments for heart failure should be initiated in patients with MINOCA.
INVASIVE TREATMENT – STENTING OF NON-OBSTRUCTIVE CULPRIT LESIONS
There is a paucity of data on the effectiveness of percutaneous coronary intervention (PCI) (with or without drug-eluting stents) for the treatment of culprit MINOCA lesions due to plaque ulceration or erosion. For this reason, dedicated working groups have not encouraged PCI of MINOCA lesions4,5.
In a pilot study, Prati et al compared the effectiveness of medical (DAPT) versus angioplasty and stenting in 31 patients with OCT-detected culprit plaque erosion92. At a median follow-up of 753 days, all patients were asymptomatic. This study was the first to suggest an alternative treatment strategy for patients with acute coronary events and non-obstructive lesions. The EROSION study confirmed this finding. Medical treatment with DAPT, and without PCI, led to an acceptable one-year revascularisation rate of 5.7%93.
In an OCT study, Imola et al evaluated the role of stent positioning for the treatment of ambiguous/intermediate lesions in 40 patients94. Drug-eluting stent treatment was performed per protocol in the presence of local thrombosis due to ulceration or erosion. Over a mean period of 4.6±3.2 months, no deaths, acute myocardial infarctions or stent thromboses were reported.
Variant angina is a diffuse coronary disease that tends to occur at different coronary sites. PCI with stenting should not be performed for vasospastic angina without severe organic stenosis95. However, stenting represents a feasible treatment for patients with vasospastic angina refractory to medical therapy95,96,97.
TREATMENT OF SCAD
In the presence of SCAD, stenting is associated with an increased risk of complications50,64,68,70, as it may propagate the vessel dissection upstream and downstream. Therefore, a conservative approach without angioplasty is recommended in the majority of patients. PCI is suggested only in the presence of high-risk anatomical features including severe proximal locations in the left main artery and left anterior descending artery, low Thrombolysis In Myocardial Infarction (TIMI) grade, or ongoing ischaemia with haemodynamic instability51,98. The difficulties inherent to the procedures, the presence of a healing process, and the frequent distal location of SCAD support this approach. Several different strategies have been utilised for PCI treatment of SCAD although there are no head-to-head studies to prove the superiority of one approach over the others. Positioning of current-generation drug-eluting stents is a common technical solution. In the absence of overlying atherosclerotic plaques, the use of bioabsorbable scaffolds seems an appealing technique99. Dilatation with cutting balloons (with or without stenting) can be a reasonable option to fenestrate the intramural haematoma and enlarge the true vessel lumen100.
Coronary artery bypass grafting (CABG) does not represent an appropriate solution in most patients with SCAD. In fact, the long-term patency of bypass grafts is poor due to the frequent healing of grafted arteries, which may lead to competitive flow and subsequent graft occlusion68.
Long-term treatment with beta-blockers is a reasonable option98. Data from a large cohort showed a lower risk of recurrences in SCAD survivors taking beta-blockers55. The adoption of lipid-lowering therapies does not seem to have much rationale for a clinical condition unrelated to cholesterol deposition. Retrospective data from the Mayo Clinic group even suggest a slightly higher risk of recurrence in patients taking statins70. Since thrombus does not seem to have a major role in the pathophysiology of SCAD67,72, the use of antiplatelet therapies remains controversial. There is a trade-off between the treatment of any existing luminal thrombosis and the risk of an extension of intramural dissection due to intramural bleeding. Based on an expert consensus, DAPT may be considered during the acute phase of SCAD and for up to one year for patients who did not undergo PCI53. Aspirin may be effective for prevention of thrombotic complications in patients with fibromuscular dysplasia but should be avoided in patients at risk of bleeding.
Conclusions and future areas of research
Knowledge of the incidence, risk factors and prognosis of MINOCA, as well as of underlying pathophysiological mechanisms, has developed significantly over the last decade. Non-invasive imaging, particularly CT scanning, may in the future become a frontline gatekeeper for non-invasive diagnosis of MINOCA. However, improved accuracy of CT will be required for the diagnosis of complicated plaques with thrombi. The application of software for the automated interpretation of intracoronary imaging modalities, especially OCT, may represent a further option to improve the diagnosis of MINOCA in routine care.
There are still fundamental gaps in our knowledge of this heterogeneous entity. The interpretation of the research literature is hampered by the different definitions of MINOCA used, the often small and selected study populations, lack of appropriate comparison populations, and adequately sized randomised clinical trials. Properly designed prospective studies are needed to gain more insight into the pathophysiology of MINOCA and into the effectiveness of medical therapies in the different pathophysiological facets of MINOCA.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

