Abstract
Background: Transcatheter aortic valve replacement (TAVR) in non-calcified aortic regurgitation (NCAR) is an off-label procedure. The balloon-expandable Myval includes extra-large sizes (30.5 mm and 32 mm) of interest in this setting.
Aims: We aimed to evaluate the safety and feasibility of Myval in NCAR.
Methods: This was an international, multicentre, observational study that enrolled all consecutive patients with symptomatic severe NCAR undergoing TAVR with the Myval device. The images were centrally analysed.
Results: A total of 113 patients were recruited, 64.6% were men, the mean age was 78.4±7.5 years, and the Society of Thoracic Surgeons score was 2.7±1.7%. Aortic root dilatation was present in 59.3% of patients, 7.1% were bicuspid, and the mean annular area was 638.6±106.0 mm2. The annular area was beyond the recommended range for extra-large sizes in 2.6% of cases, and additional volume was added in 92% (median 4 cc, up to 9 cc). The extra-large sizes were used in 95 patients (84.1%), and the mean oversizing was 17.9±11.0%. The technical success rate was 94.7%; the rate of residual ≥moderate aortic regurgitation was 8.9%, and the pacemaker rate was 22.2%. There were no cases of annular rupture, cardiac tamponade, or aortic dissection, but in 4 patients (3.5%) valve embolisation occurred (1 antegrade and 3 ventricular), all in cases with a tapered left ventricle outflow tract (p=0.007). Thirty-day and 1-year mortality were 5.3% and 9.7%, respectively. Technical success was associated with better survival (97.1% vs 72.7%; p=0.012), and valve embolisation was the main determinant of mortality (p=0.047).
Conclusions: Myval is a feasible and safe option for selected non-operable patients with NCAR and demonstrated good midterm outcomes and lack of impact of oversizing on device durability.
Introduction
The rapidly growing worldwide experience in transcatheter aortic valve replacement (TAVR) is leading to more frequent use in off-label scenarios such as non-calcified aortic regurgitation (NCAR)123. Despite promising results with newer-generation devices in NCAR4, the procedures are technically challenging because of the absence of calcified native valves to anchor the prosthetic device to, the larger annuli, and the greater degree of oversizing required which might increase the risk of complications56.
A recent meta-analysis of 11 studies, including 911 patients undergoing TAVR for NCAR, demonstrated a device success rate of 80.4%, ≥moderate aortic regurgitation (AR) in 7.4% of patients, and a 30-day mortality rate of 9.5%, with up to 3% requiring conversion to open surgery7. These rates compare unfavourably with the results of TAVR in aortic stenosis (AS). Although a dedicated device for NCAR has been designed – the Trilogy TAVR system (JenaValve Technology) – the current version does not cover annuli with diameters greater than 27 mm89.
The Myval balloon-expandable TAVR device (Meril Life Sciences Pvt. Ltd.) offers the potential advantage of covering the largest range of annular areas (up to 840 mm2 at nominal inflation volume, 32.7 mm diameter). However, only anecdotal cases have been reported to date10, and long-term results – particularly after supra-nominal volume inflation – are unknown. Therefore, we aimed to research the range of experiences with the Myval TAVR device and report the acute and midterm outcomes.
Methods
Study design and patient population
This was an international, multicentre, observational study that enrolled all consecutive patients with symptomatic severe NCAR undergoing TAVR. The registry was initiated in January 2019, and a total of 17 centres in Europe, the USA, and the Asia-Pacific region participated. Patients were considered candidates for the procedure if they had severe NCAR with comorbid conditions that would preclude surgical valve replacement according to each centre’s Heart Team meeting. Patients with aortic stenosis, defined as a peak aortic jet velocity on continuous-wave Doppler of >2.5 m/s, were excluded from this study. We collected data retrospectively for cases performed before initiation of the study and prospectively thereafter.
This study was approved by the institutional review board of each institution, and all patients provided written informed consent for TAVR and the use of anonymous clinical, procedural, and follow-up data for research. For the retrospective analyses of clinically acquired and anonymised data, the institutional review boards of some of the participating institutions waived the need for written patient informed consent.
Study device and TAVR procedure
Patients were selected for TAVR at the institutional level after discussions by the multidisciplinary Heart Team. Device size was selected based on three-dimensional computed tomography. The access site and type of device were determined by the multidisciplinary Heart Team. All TAVR procedures were conducted in accordance with local guidelines using standard techniques via transfemoral access, and the balloon-expandable transcatheter valve Myval11 was implanted.
Data collection
Data collection included baseline clinical, laboratory, echocardiographic, and computed tomographic data, as well as procedural data and clinical follow-up data at prespecified timepoints (1 and 12 months and yearly thereafter, as long as available).
Follow-up was obtained through clinical visits and/or by telephone, and information about cause of death and rehospitalisation was collected. Referring cardiologists, general practitioners, and patients were contacted whenever necessary for further information. All data provided by each institution were anonymised and centrally collected, and all inconsistencies were resolved directly with local investigators and onsite data monitoring.
Endpoints and definitions
The primary endpoints of the present study were all-cause and cardiovascular mortality rates at 1 year. Secondary endpoints were rehospitalisation, device success, and other 30-day major clinical endpoints defined according to the Valve Academic Research Consortium-3 (VARC-3) criteria12. Other endpoints included procedure- and device-related complications and a postprocedural echocardiographic assessment of the valve and cardiac function. Baseline and postprocedural echocardiographic tests were evaluated in a core laboratory (www.icicorelab), and angiographic images of the procedures were obtained for all cases; baseline computed tomography was centrally analysed in 89.3% of the population as well. The severity of AR was qualitatively assessed and graded using transthoracic echocardiography according to established guidelines and VARC-3 criteria12. The perimeter and area oversizing indices were defined as follows: [(device nominal perimeter or area)/(annulus perimeter or area measured by computed tomography)] x100, respectively.
Statistical analysis
Continuous variables are presented as mean±standard deviation and were compared using the Student’s t-test or Mann-Whitney U test. Categorical variables are presented as counts or percentages and were compared using the chi-square or Fisher’s exact test. Receiver operating characteristic curve analysis was performed, and areas under the curve were calculated to assess the discriminative powers of device-sizing parameters for postprocedural AR ≥moderate. The cumulative rates of death or rehospitalisation were calculated using the Kaplan-Meier survival analysis. For rehospitalisation, data were censored at the time of death or the end of the observation period. Multivariate analysis was not performed to avoid overfitting due to the reduced number of events. All statistical analyses were performed using R software, version 3.6.1 (R Project for Statistical Computing). A 2-sided p-value of <0.05 was considered as statistically significant.
Results
Baseline characteristics
A total of 113 patients were analysed, 73 (64.6%) were men, the mean age was 78.4±7.5 years, and the Society of Thoracic Surgeons (STS) and European System for Cardiac Operative Risk Evaluation (EuroSCORE) II scores were 2.7±1.7% and 3.5±2.7%, respectively. The reasons that led the local Heart Teams to exclude surgery included the following: critical pre-operative status in 14 patients (12.4%); left ventricular function below 30% in 42 patients (37.2%); prior cardiac surgery in 6 patients (5.3%); severe comorbidities in 46 patients (40.7%); and a high-risk score plus patient preference in 5 cases (4.4%). The main baseline clinical and anatomical characteristics are described in Table 1.
Most patients were in New York Heart Association (NYHA) Functional Class II (31.9%) or III (54%), but the procedures were considered urgent/emergent in 12.4% of the study population. There were no cases of AS (mean transvalvular gradient 6.31±3.52 mmHg and a maximum transvalvular gradient of 18 mmHg in 1 patient), and the degree of AR was severe in 96.5% of patients after core lab assessment; 59.3% had aortic root dilatation ≥40 mm, and 7.1% were bicuspid. The mean annular area was 638.6±106.0 mm2 with no calcification in 85% and minor calcification in 15% of cases, including leaflet calcification (14.1%) and extension to the left ventricular outflow tract (LVOT; 0.9%). The median Agatston score was 0 Hounsfield units (HU) (mean of 56.5 HU, with 995 HU as the highest value). The subannular shape of the LVOT was tubular in 12.4% of cases, flared in 58.4%, and tapered in 28.6%.
Table 1. Baseline clinical and anatomical characteristics.
| Clinical characteristics | N=113 | |
|---|---|---|
| Age, years | 78.4±7.46 | |
| Male | 73 (64.6) | |
| BMI, kg/m2 | 27.3±4.7 | |
| Hypertension | 97 (85.8) | |
| Diabetes | 30 (26.5) | |
| GFR <30 ml/min/1.73 m2 | 18 (15.9) | |
| Cirrhosis | 2 (1.8) | |
| Prior myocardial infarction | 6 (5.3) | |
| Prior PCI | 6 (5.3) | |
| Prior CABG | 4 (3.5) | |
| Prior cardiac surgery | 6 (5.3) | |
| Prior pacemaker | 12 (10.6) | |
| Peripheral artery disease | 11 (9.7) | |
| Atrial fibrillation | 35 (31.0) | |
| Prior stroke | 8 (7.1) | |
| COPD | 25 (22.1) | |
| NYHA Class III-IV | 71 (62.8) | |
| Urgent preoperative state | 14 (12.4) | |
| STS score | 2.71±1.7 | |
| EuroSCORE II | 3.48±2.7 | |
| Echocardiographic measurements | ||
| Baseline LVEF, % | 43.4±14.7 | |
| Baseline LVEDD, mm | 63.6±9.2 | |
| Aortic valve mean pressure gradient, mmHg | 6.3±3.5 | |
| Computed tomography findings | ||
| Annulus area, mm2 | 638.5±106.1 | |
| Annulus perimeter, mm | 88.5±8.0 | |
| LMCA height, mm | 14.8±3.6 | |
| RCA height, mm | 17.4±3.8 | |
| Agatston units, HU | 56.4±159.5 | |
| STJ mean diameter, mm | 37.0±5.6 | |
| SoV mean diameter, mm | 39.15±6.0 | |
| Leaflet calcification | 17 (15.0) | |
| LVOT calcification | 1 (0.9) | |
| LVOT shape | Tubular | 14 (12.4) |
| Flared | 66 (58.4) | |
| Tapered | 32 (28.3) | |
| Horizontal aorta | 4 (3.5) | |
| Bicuspid aortic valve | 8 (7.1) | |
| Data are presented as n (%) or mean±standard deviation. BMI: body mass index; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; CT: computed tomography; EuroSCORE: European System for Cardiac Operative Risk Evaluation; GFR: glomerular filtration rate; HU: Hounsfield units; LMCA: left main coronary height; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; RCA: right coronary artery; SoV: sinus of Valsalva; STJ: sinotubular junction; STS: Society of Thoracic Surgeons | ||
Procedural and in-hospital outcomes
As summarised in Table 2, all the procedures were performed through the transfemoral approach, under general anaesthesia in 39.8% of the cases and under transoesophageal guidance in 19.5%. No predilation sizing was used except in 1 case, and post-dilation was required in 5 cases (4.4%), adding 2 cc and 3 cc of extra volume to the balloon in 4 and 1 cases, respectively. The valve annular area was within the range recommended for extra-large sizes in 81.4% of cases, and additional volume was added to the balloon in 92% of cases with a median of 4 cc (±2.3) and up to 9 cc in 3 cases. The distribution of the degree of oversizing is depicted in Figure 1. Supplementary Table 1 shows the degree of oversizing according to the extra volume added to the 32 mm Myval device. The mean oversizing was 17.9±11.0%; this varied from â9.5 to 38.1% across the study population. Technical and procedural success rates were 94.7% and 92.0%, respectively. There were no cases of annular rupture, cardiac tamponade, coronary obstruction, or stroke, but in 4 cases (3.5%), valve embolisation occurred (1 antegrade and 3 ventricular). Antegrade embolisation was solved with a second valve implantation. Regarding the 3 cases of ventricular embolisation, a tapered LVOT was present in all of them, and this morphology was significantly related to the risk of embolisation (12.5% vs 0%; p=0.007) (Central illustration). One ventricular embolisation required surgical repair, performed 24 hours after the procedure since it was an incidental finding in the echocardiography performed the day after TAVR; the other 2 cases of ventricular embolisation were solved with a balloon inflation within the prosthesis in the left ventricle and removal of the valve to the descending aorta by gently pulling (Supplementary Figure 1, Moving image 1). In both cases, the implantation of a second valve of a larger size was successfully achieved. In one of the two cases, however, the patient, who had a left ventricular ejection fraction of 15%, died shortly after the procedure due to acute heart failure.
Postprocedural echocardiography demonstrated no residual AR in 68.1%, trivial AR in 23%, mild in 7.1% and moderate in 1.8% of cases. A permanent pacemaker implantation was required in 13.3% of the population before hospital discharge. The procedure was considered a technical success, according to VARC-3 criteria, in 94.7% of cases. In-hospital mortality was 3.5%, and cardiovascular mortality was 1.8%. No cerebrovascular events occurred.
Table 2. Procedural and in-hospital characteristics.
| Procedural characteristics | N=113 | |
|---|---|---|
| General anaesthesia | 45 (39.8) | |
| Intraprocedural TOE | 22 (19.5) | |
| Transfemoral access | 113 (100) | |
| Concomitant PCI | 0 (0) | |
| Contrast, ml | 99.6±31.7 | |
| Procedural time, min | 53.4±10.5 | |
| Fluoroscopy time, min | 8.2±2.0 | |
| Predilatation | 1 (0.9) | |
| Post-dilatation | 5 (4.4) | |
| Annular rupture | 0 (0) | |
| Coronary obstruction | 0 (0) | |
| Cerebral protection device | 1 (0.9) | |
| Procedural death | 1 (0.9) | |
| VARC-3 technical success | 107 (94.7) | |
| First valve size | 23 mm | 1 (0.9) |
| 24.5 mm | 1 (0.9) | |
| 26 mm | 2 (1.8) | |
| 27.5 mm | 3 (2.7) | |
| 29 mm | 11 (9.7) | |
| 30.5 mm | 14 (12.4) | |
| 32 mm | 81 (71.7) | |
| Balloon with more than nominal volume | 80 (70.8) | |
| Volume in the prosthesis balloon | Nominal | 26 (24.5) |
| +1 cc | 14 (13.2) | |
| +2 cc | 15 (14.2) | |
| +3 cc | 21 (19.8) | |
| +4 cc | 8 (7.5) | |
| +5 cc | 4 (3.8) | |
| +6 cc | 7 (6.6) | |
| +7 cc | 1 (0.9) | |
| +8 cc | 9 (8.5) | |
| +9 cc | 1 (0.9) | |
| % of oversizing | 17.9±11.0 | |
| Mean extra cc in the prosthesis balloon | 2.7±2.5 | |
| Need for a 2nd valve implantation | 4 (3.5) | |
| Valve embolisation | 4 (3.5) | |
| Ventricular embolisation | 3 (2.7) | |
| Antegrade embolisation | 1 (0.9) | |
| Embolisation management | Conversion to surgery | 1 (25.0) |
| Balloon pullout of 1st valve + 2nd valve implantation | 3 (75.0) | |
| In-hospital outcomes | ||
| Residual aortic regurgitation (moderate-severe) | 10 (8.9) | |
| All-cause mortality | 4 (3.5) | |
| Cardiovascular mortality | 2 (1.8) | |
| Stroke | 0 (0) | |
| TIA | 0 (0) | |
| Major vascular complications | 2 (1.8) | |
| Permanent pacemaker implantation | 15 (13.4) | |
| Myocardial infarction | 0 (0) | |
| Major bleeding | 1 (0.9) | |
| AKI | 4 (3.5) | |
| Grade 1 | 3 (2.6) | |
| Grade 2 | 1 (0.9) | |
| Length of in-hospital stay, days | 8.2±7.0 | |
| Data are presented as n (%) or mean±standard deviation. AKI: acute kidney injury; cc: cubic centimetre; LVOT: left ventricular outflow tract; PCI: percutaneous coronary intervention; TIA: transient ischaemic attack; TOE: transoesophageal echocardiogram; VARC-3: Valve Academic Research Consortium-3 | ||
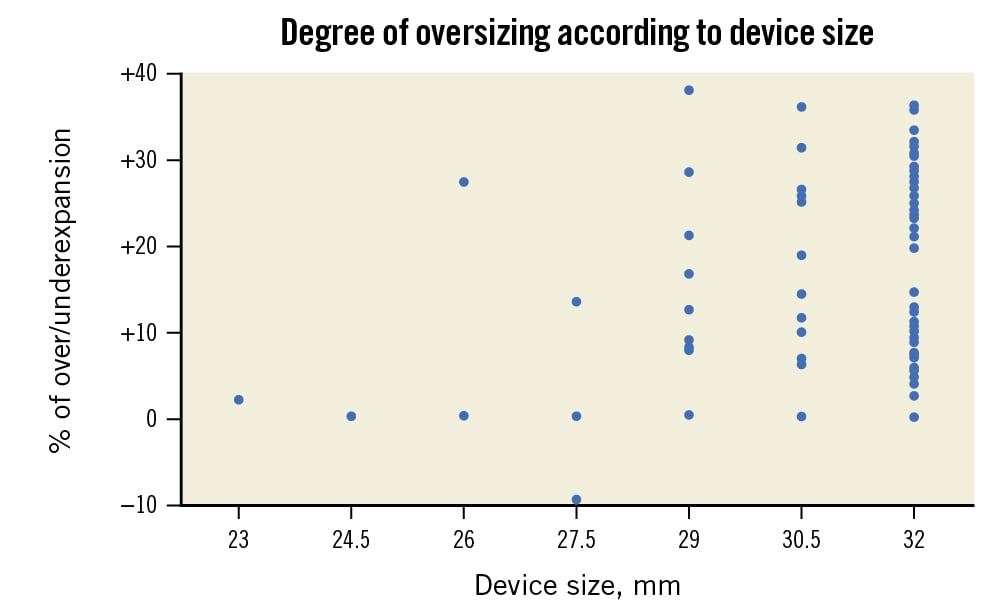
Figure 1. Degree of oversizing performed according to device size.
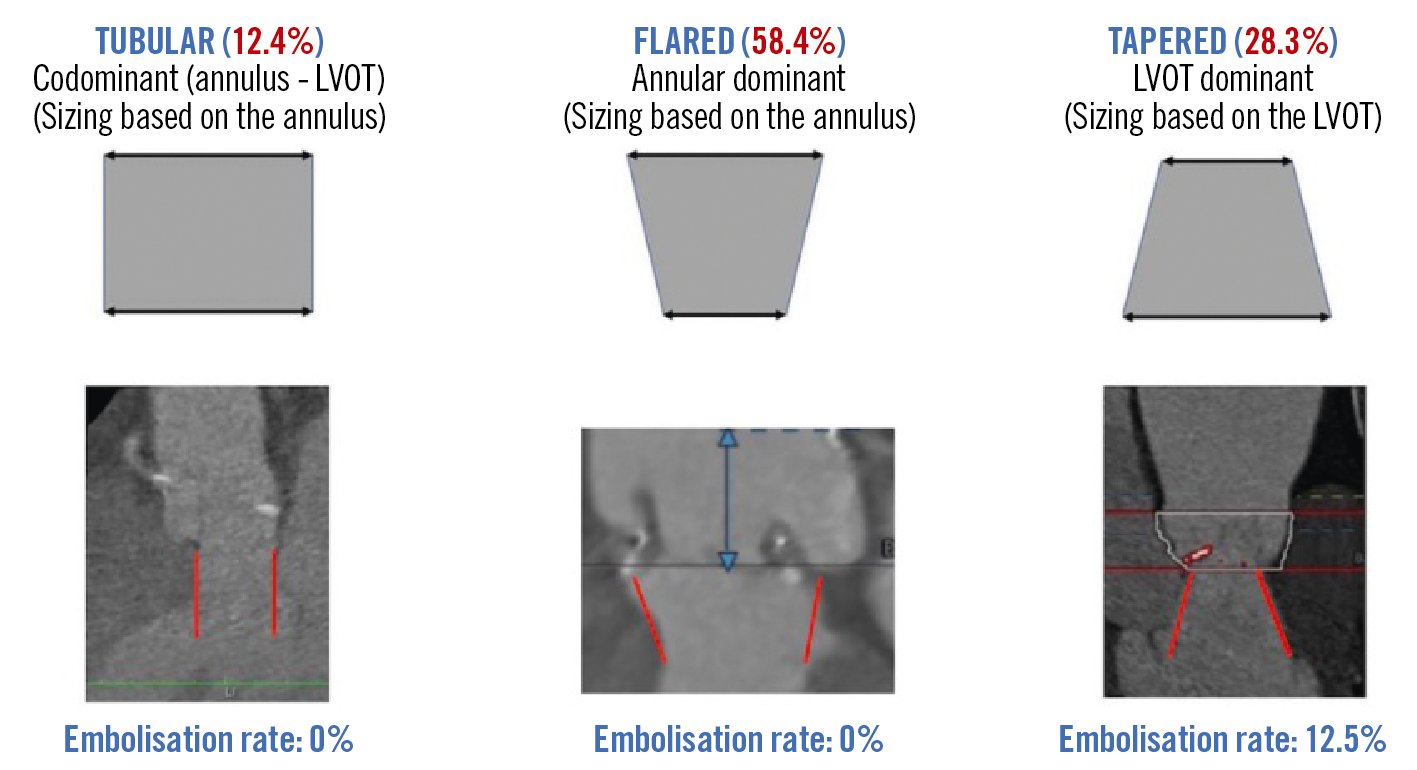
Central illustration. Rate of device embolisation according to the morphology of the left ventricular outflow tract. Different LVOT shapes were identified by computed tomography. The most common shape was flared (centre) and less common was a tubular LVOT (left). Neither of these was complicated by valve embolisation. On the other hand, four cases of valve embolisation occured when the LVOT was tapered (right). LVOT: left ventricular outflow tract
Thirty-day and midterm clinical outcomes
At 30 days, there were 2 more deaths, leading to a global mortality rate of 5.3%, and there were 2 cerebrovascular events (1.8%) (Table 3). The need for a permanent pacemaker was 15.1%, and the need for rehospitalisation was 3.5%. The degree of residual aortic regurgitation is summarised in Figure 2.
The 1-year follow-up global mortality rate (9.7%) and the combined endpoint of mortality and rehospitalisation are presented in Figure 3. Cerebrovascular events occurred in 5.3% of the patients, and the global pacemaker rate increased to 22.2%. The main factors associated with 1-year mortality are presented in Supplementary Table 2. The only anatomical factor that predicted a higher mortality rate was a larger end-diastolic left ventricular diameter (62.0±3.4 vs 57.8±7.9 mm; p=0.035). Technical success was associated with better survival (97.1% vs 72.7%; p=0.012), and valve embolisation was the main determinant of mortality (p=0.047), despite being resolved in 3 out of 4 cases during the procedure. Residual regurgitation was not associated with increased mortality.
Regarding the risk for mortality and rehospitalisation at 1 year, the main predictors, as reflected in Supplementary Table 3, included technical failure, the left ventricular ejection fraction at follow-up (31.0±16.9% in those who died at 1 year vs 43.2±13.0% in survivors; p=0.007) and gender − with greater mortality in men (20.5%) than in women (5.0%; p=0.027) and larger aortic annuli in men (692±170 mm2) than in women (610±101 mm2; p=0.041).
The mean follow-up was 762 (interquartile range 391-811) days, with 1-year follow-up, available in most patients (84.3%), demonstrating correct prosthesis functioning despite large oversizing, but only half of the study population completed the 3-year follow-up, precluding further assessment.
Table 3. Thirty-day and 1-year outcomes.
| 30-day outcomes | N=113 |
|---|---|
| All-cause mortality | 6 (5.3) |
| Stroke | 1 (0.9) |
| TIA | 1 (0.9) |
| Myocardial infarction | 4 (3.5) |
| Permanent pacemaker implantation | 17 (15.0) |
| Rehospitalisation for heart failure | 4 (3.5) |
| LVEF, % | 41.6±14.9 |
| LVEDD, mm | 64.0±8.8 |
| Aortic valve mean pressure gradient, mmHg | 4.7±2.0 |
| Residual aortic regurgitation (moderate-severe) | 10 (9.1) |
| Device success | 107 (94.7) |
| 1-year follow-up | |
| All-cause mortality | 11 (9.7) |
| Stroke | 2 (1.8) |
| TIA | 2 (1.8) |
| Permanent pacemaker implantation | 25 (22.1) |
| Rehospitalisation for heart failure | 9 (8.0) |
| Residual aortic regurgitation (moderate-severe) | 9 (8.0) |
| Data are presented as n (%) or mean±standard deviation. LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; TIA: transient ischaemic attack | |
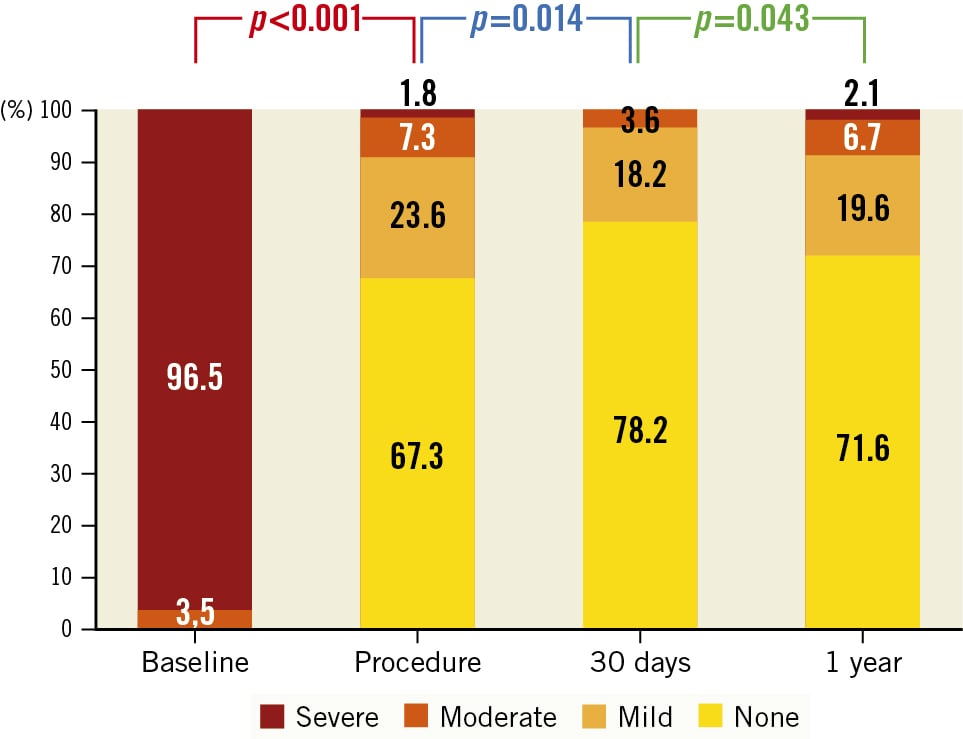
Figure 2. Degree of aortic regurgitation from baseline to 1-year follow-up.
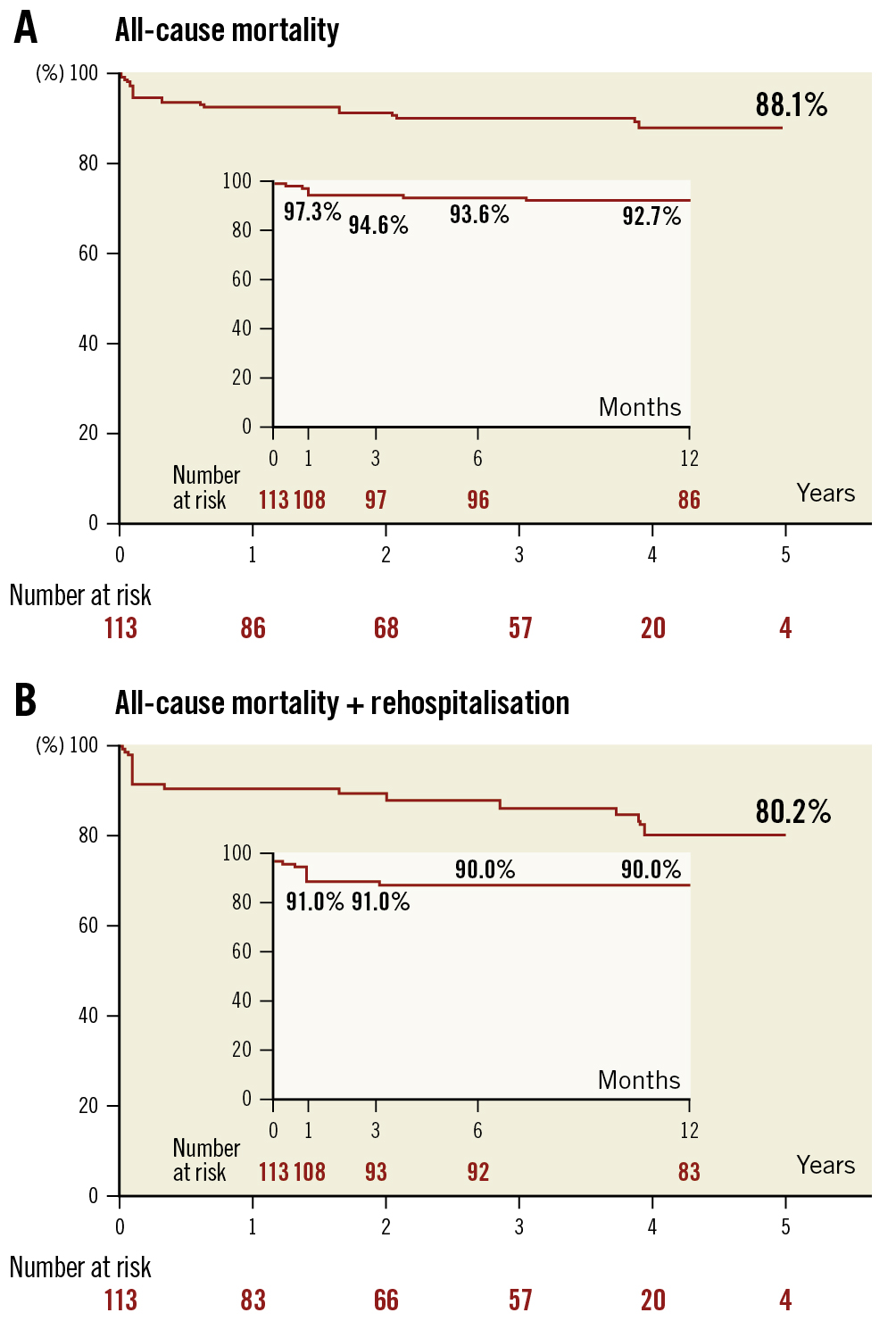
Figure 3. Survival curves for 5-year mortality and 5-year mortality + rehospitalisation. A) Survival curves for 5-year mortality; B) Survival curves for 5-year mortality + rehospitalisation.
Discussion
This is the first systematic registry on the use of the balloon-expandable Myval device for the treatment of NCAR. Although this is a non-dedicated device and its use therefore remains off-label in NCAR, it has become the preferred option in those centres where it is available. This is because of the extra-large sizes it offers which are the only ones available for the large annuli often present in patients with this condition. The main findings of our study are 1) large sizes were used in 80% of the patients, and the addition of up to 9 cc of extra volume to the balloon was not related to short- or midterm clinical or structural complications; 2) procedural success rate was high (92%) and similar to that reported in contemporary registries with newer-generation devices (90.2%)7 but with better results in terms of residual ≥moderate AR rate (1.8%) and a low rate of severe complications; 3) valve embolisation occurred in 3.5% of the cases, mainly in the presence of tapered LVOT, and was associated with increased mortality despite adequate technical resolution in most of the cases; 4) the 30-day global mortality rate was 5.3%, and 1-year mortality was 9.7%, which compares favourably with those reported in a recent meta-analysis (6.1% and 11.8%, respectively)7; however, at 1 year, the need for new permanent pacemaker was high (22.2%).
Relevance of non-calcified aortic regurgitation
AR is the reverse blood flow from the aorta into the left ventricle (LV) during diastole, and it results from malcoaptation of the aortic leaflets due to abnormalities of the aortic leaflets, their supporting structures, such as the aortic root and annulus, or both13. This causes left ventricular volume overload, dilatation and eccentric hypertrophy, which in turn, allows ejection of a larger stroke volume. However, over time, it results in a decline in systolic function and ejection fraction1314. Although less prevalent than AS, it remains a frequently encountered clinical problem in the adult population, with an estimated prevalence of at least moderate AR in 2% of patients older than 70 years1415. Once symptoms related to AR develop, the prognosis becomes worse with a 10-20% annual mortality rate15. According to the current European and American guidelines, surgical intervention is indicated when significant AR is accompanied by symptoms, by decreased left ventricular systolic function with a diminished left ventricular ejection fraction (LVEF) or severe dilatation1617. However, many patients are inoperable because of advanced age or comorbidities, and safe, effective non-surgical options remain an unmet need with TAVR being considered feasible as a bailout option for high-risk or inoperable patients, as reported in a meta-analysis by Franzone et al18. Since TAVR is a challenging intervention in these cases due to the anatomical complexity and the lack of dedicated devices, it is only performed in carefully selected patients as an off-label indication with unpredictable immediate and long-term results1920. On the other hand, when left untreated, these patients face an annual mortality risk of 20%, confirming the unmet need of a less invasive approach2021.
Percutaneous treatment of pure AR
There are anatomical and haemodynamic factors which confer complexity to transcatheter procedures19. The main anatomical factors, compared to AS, include larger aortic annuli which, due to the current limited availability of adequately sized devices, can lead to significant postprocedural residual AR1920. There is often also an aortic root dilatation which makes device positioning and deployment more difficult. As well, the absence of sufficient annular and/or leaflet calcification results in an inadequate valve anchoring in the annulus and, thus, reduces stability during the valve deployment20. In addition, the morphology of the LVOT-aorta might be predisposed to a higher risk of valve embolisation; this has been previously described for bicuspid aortic valve stenosis and was confirmed in our series for those patients with a tapered LVOT, suggesting that the threshold of the aortic annular area might differ according to LVOT morphology − as depicted in the Central illustration.
Regarding the haemodynamic factors that may influence the outcome of the procedure, compared to AS, typically in NCAR, there is a larger stroke volume and a “suction-effect” which makes the positioning and deployment of an eventual transcatheter valve more difficult, less precise and, therefore, unpredictable.20 This can then lead to embolisation or significant postprocedural residual AR, which, in turn, are associated with worse clinical outcomes and mortality21.
The feasibility of TAVR in NCAR using non-dedicated devices approved only for the treatment of AS has been shown in many published case series and registries47. The first-in-human reports and case series that suggested the feasibility and safety of TAVR in NCAR involved the compassionate use of the CoreValve/Evolut (Medtronic) or SAPIEN (Edwards Lifesciences) systems2122. A multicentre study by De Backer et al showed that TAVR is a feasible treatment strategy in selected high-risk patients with NCAR but is associated with a considerable risk of device malpositioning and residual AR, although newer-generation TAVR devices were associated with better outcomes23. Similarly, another multicentre study by Yoon et al found that, compared with the early-generation devices for TAVR, the new-generation devices were associated with improved procedural outcomes in treating patients with NCAR4. Likewise, in an international registry, Sawaya et al reported that treating NCAR with TAVR devices is challenging but feasible and that, while the use of early-generation devices was associated with device embolisation or migration and significant residual regurgitation, there were promising outcomes with new-generation devices24. Finally, a meta-analysis by Hi et al reported that, compared with the early-generation devices, the use of new-generation devices was associated with significantly higher device success rates and notably lower rates of second-valve deployment, significant residual AR and all-cause mortality7. However, it remains unclear which is the best device in this setting; although the self-expanding Evolut has the theoretical advantage of offering larger sizes, as well as being resheathable and repositionable, the current results comparing it with the SAPIEN series suggest a greater need for a second valve (18.0% Evolut vs 12.2% SAPIEN 3), lower device success (76.0% Evolut vs 85.4% SAPIEN 3), higher postprocedural significant AR (4% Evolut vs 0% SAPIEN 3), and greater need for a definitive pacemaker (21.4% Evolut vs 18.2% SAPIEN 3)4. Therefore, balloon-expandable technologies are a more attractive alternative for current cases with NCAR accepted for transcatheter treatment, but, as oversizing is the only anchoring mechanism, several concerns exist regarding the short- and long-term impact.
Oversizing balloon-expandable TAVR devices in NCAR
TAVR procedures for NCAR are technically challenging because of the large annuli and the absence of a calcified native valve to anchor the prosthetic device to, and therefore the required greater degree of oversizing, which might increase the risk of complications. Although there are no official recommendations, 20-30% oversizing is often used. Beyond the increased risk of conduction system abnormalities leading to greater permanent pacemaker implantation25, there remains a crucial question as to whether or not this oversizing might increase the risk of annular rupture or aortic dissection20. Although the increased risk for permanent pacemaker implantation secondary to valve oversizing is considerable, due to the lack of annular and/or leaflet calcification, this approach is essential for anchoring the valve and therefore, avoiding embolisation. It is known that if there is left ventricular dysfunction after TAVR for aortic stenosis, the use of a pacemaker has been associated with low rates of significant recovery but not with increased mortality26. It remains unknown if the prognostic impact in the setting of AR would be the same; on the other hand, currently more physiological pacing strategies are being used and may help to improve the outcomes. Myval is a balloon-expandable TAVR system that has shown favourable clinical outcomes in terms of safety, rate of permanent pacemaker implantation, transvalvular gradients and residual paravalvular regurgitation in aortic stenosis1127. Since the device is manufactured in standard sizes (20 mm, 23 mm, 26 mm and 29 mm), as well as intermediate (21.5 mm, 24.5 mm, 27.5 mm) and extra-large sizes (30.5 mm and 32 mm), it allows for a more precise degree of oversizing and covers aortic annular perimeters/areas of up to 100.5 mm and 840 mm2, respectively28. Ancona et al reported the first successful case using an extra-large size Myval BEV (32 mm) in a patient with severe NCAR secondary to a left ventricular assist device (Moving image 2 shows another case example with the same indication), confirming that using a non-dedicated extra-large size balloon-expandable TAVR device was feasible and safe despite large oversizing10. Importantly, it is known that the diameter, perimeter, and the area of the aortic annulus only provide the same sizing recommendation in about 60% of cases. In this study, aortic annuli areas were used as the main reference, but according to Horehledova et al29, larger prostheses might be selected if based on perimeter dimensions; this fact might be particularly relevant for NCAR. In this regard, although non-contrast-enhanced acquisition might not be routinely performed in some institutions during computed tomography analysis performed before TAVR for aortic stenosis, a preliminary acquisition without contrast is recommended for pure AR planification because the presence of calcium – even small amounts – can be determinant in whether a patient is accepted for TAVR and for selecting the adequate size in borderline dimensions.
Finally, large overexpansion might have an impact on the durability of the device; although, bench tests demonstrated that no acute damage occurred with the former-generation SAPIEN XT, the findings by Basman et al28 and Miyasaka et al30 for extra-large annuli are reassuring and in line with our findings, suggesting that prosthesis overexpansion is safe, as confirmed in our series at least at 1-year follow up.
Limitations
The main limitations of our research are based on its retrospective nature despite the central analysis of images. The relatively small sample size in selected centres and the current-generation Myval device – but not the newer iterations – may have limited the assessment of procedural success rates and complications. However, the present registry is the first to gather a contemporary cohort of patients treated with the new Myval device that covers the largest range of aortic annuli. Finally, the potential role of new, dedicated devices for NCAR was not considered, since they were not available in the participating institutions.
Conclusions
Although dedicated devices have a promising future in TAVR for severe NCAR, the current-generation non-dedicated devices will remain the only alternative for these patients over the next few years. Our registry suggests that for selected patients with NCAR who are not considered surgical candidates, TAVR with the balloon-expandable Myval device, and in particular, its extra-large sizes, represents a safe and feasible option, given the options for greater oversizing than for aortic stenosis cases. The procedural success rate was high, residual moderate or severe regurgitation was low, and adequate 1-year survival with no cases of early prosthesis deterioration was confirmed.
Impact on daily practice
The adequate outcomes of the novel balloon-expandable Myval device for the treatment of NCAR confirms the safety and feasibility of this new alternative for the treatment of a challenging subset of patients. Moreover, the use of a large degree of oversizing was demonstrated to be a valid strategy for device anchoring with no cases of annular rupture or aortic dissection. There was also no structural deterioration of the devices at 1-year follow-up despite the use of up to 9 cc of extra volume in the delivery system. Longer-term follow-up is required to confirm the integrity of the valve and the low rate of late-onset complications.
Conflict of interest statement
I.J. Amat-Santos, P. Martín, A. Ielasi, J.P. De Brahi, and P. Lamelas are proctors for Meril Life. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Example of non-calcified aortic regurgitation with ventricular embolisation of the prosthesis successfully resolved.
Moving image 2. Example of non-calcified aortic regurgitation secondary to Heartmate-3 (Abbott) successfully treated with Myval 32 mm.

