Abstract
BACKGROUND: There are limited data on the impact of transcatheter heart valve (THV) type on the outcomes of surgical explantation after THV failure.
AIMS: We sought to determine the outcomes of transcatheter aortic valve replacement (TAVR) explantation for failed balloon-expandable valves (BEV) versus self-expanding valves (SEV).
METHODS: From November 2009 to February 2022, 401 patients across 42 centres in the EXPLANT-TAVR registry underwent TAVR explantation during a separate admission from the initial TAVR. Mechanically expandable valves (N=10, 2.5%) were excluded. The outcomes of TAVR explantation were compared for 202 (51.7%) failed BEV and 189 (48.3%) failed SEV.
RESULTS: Among 391 patients analysed (mean age: 73.0±9.8 years; 33.8% female), the median time from index TAVR to TAVR explantation was 13.3 months (interquartile range 5.1-34.8), with no differences between groups. Indications for TAVR explantation included endocarditis (36.0% failed SEV vs 55.4% failed BEV; p<0.001), paravalvular leak (21.2% vs 11.9%; p=0.014), structural valve deterioration (30.2% vs 21.8%; p=0.065) and prosthesis-patient mismatch (8.5% vs 10.4%; p=0.61). The SEV group trended fewer urgent/emergency surgeries (52.0% vs 62.3%; p=0.057) and more root replacement (15.3% vs 7.4%; p=0.016). Concomitant cardiac procedures were performed in 57.8% of patients, including coronary artery bypass graft (24.8%), and mitral (38.9%) and tricuspid (14.6%) valve surgery, with no differences between groups. In-hospital, 30-day, and 1-year mortality and stroke rates were similar between groups (allp>0.05), with no differences in cumulative mortality at 3 years (log-rank p=0.95). On multivariable analysis, concomitant mitral surgery was an independent predictor of 1-year mortality after BEV explant (hazard ratio [HR] 2.00, 95% confidence interval [CI]: 1.07-3.72) and SEV explant (HR 2.00, 95% CI: 1.08-3.69).
CONCLUSIONS: In the EXPLANT-TAVR global registry, BEV and SEV groups had different indications for surgical explantation, with more root replacements in SEV failure, but no differences in midterm mortality and morbidities. Further refinement of TAVR explantation techniques are important to improving outcomes.
The expansion of transcatheter aortic valve replacement (TAVR) to younger, lower-risk patients with longer life expectancies will likely see an increase in future valve reintervention12. While redo-TAVR (transcatheter aortic valve [TAV]-in-TAV) remains an attractive option for transcatheter heart valve (THV) failure in carefully selected patients, not all patients will be eligible, due to indication or unfavourable anatomy345. On the other hand, surgical explantation of the THV (TAVR explant) can be offered to most patients who are surgical candidates, in addition to those presenting with endocarditis or concomitant pathologies that need to be addressed with open surgery. However, the reported mortality and morbidities associated with TAVR explant are not negligible, as evidenced by a recent analysis of the Society of Thoracic Surgeons (STS) database6 and our prior study from the EXPLANT-TAVR registry7. TAVR explant is also technically more challenging, unlike first-time or even redo-surgical aortic valve replacement (SAVR), with respect to lacking a clean tissue plane for TAVR removal and often involving surrounding structures which may be influenced by the type of THV explanted. The clinical impact of the THV type (balloon-expandable valve [BEV] versus self-expanding valve [SEV]) after TAVR explant remains unknown. We therefore performed an in-depth evaluation comparing patients undergoing TAVR explant for failed BEV versus failed SEV.
PATIENTS AND METHODS
DATA SOURCE
The EXPLANT-TAVR registry is a multicentre, international registry with data compiled from 42 centres worldwide and includes patients who underwent surgical explantation of a THV after TAVR during a separate hospital admission. Our study design has been previously described7. Since all participating institutions contributed cases after obtaining local institutional review board approvals, the requirement to obtain patient consent was waived. The 30-day and longer-term follow-up of all subjects in this registry were adjudicated separately by each individual institution.
PATIENT POPULATION
We retrospectively analysed data from adult patients who underwent TAVR explant between November 2009 and February 2022. Mechanisms of TAVR failure included structural valve deterioration (SVD), prosthesis-patient mismatch (PPM), endocarditis, paravalvular leak (PVL), and valve migration. The primary indication for TAVR explant and primary reasons for exclusion from redo-TAVR were systematically determined by the multidisciplinary Heart Team at each respective institution. Patients undergoing concomitant coronary artery bypass graft (CABG) or valvular procedures were included. All TAVR explants performed during the same admission as the initial TAVR procedure were excluded as well as operations for mechanically expandable valve failure (N=10, 2.5%) (Figure 1). The final study cohort was stratified into patients undergoing TAVR explant for failed BEV and failed SEV.
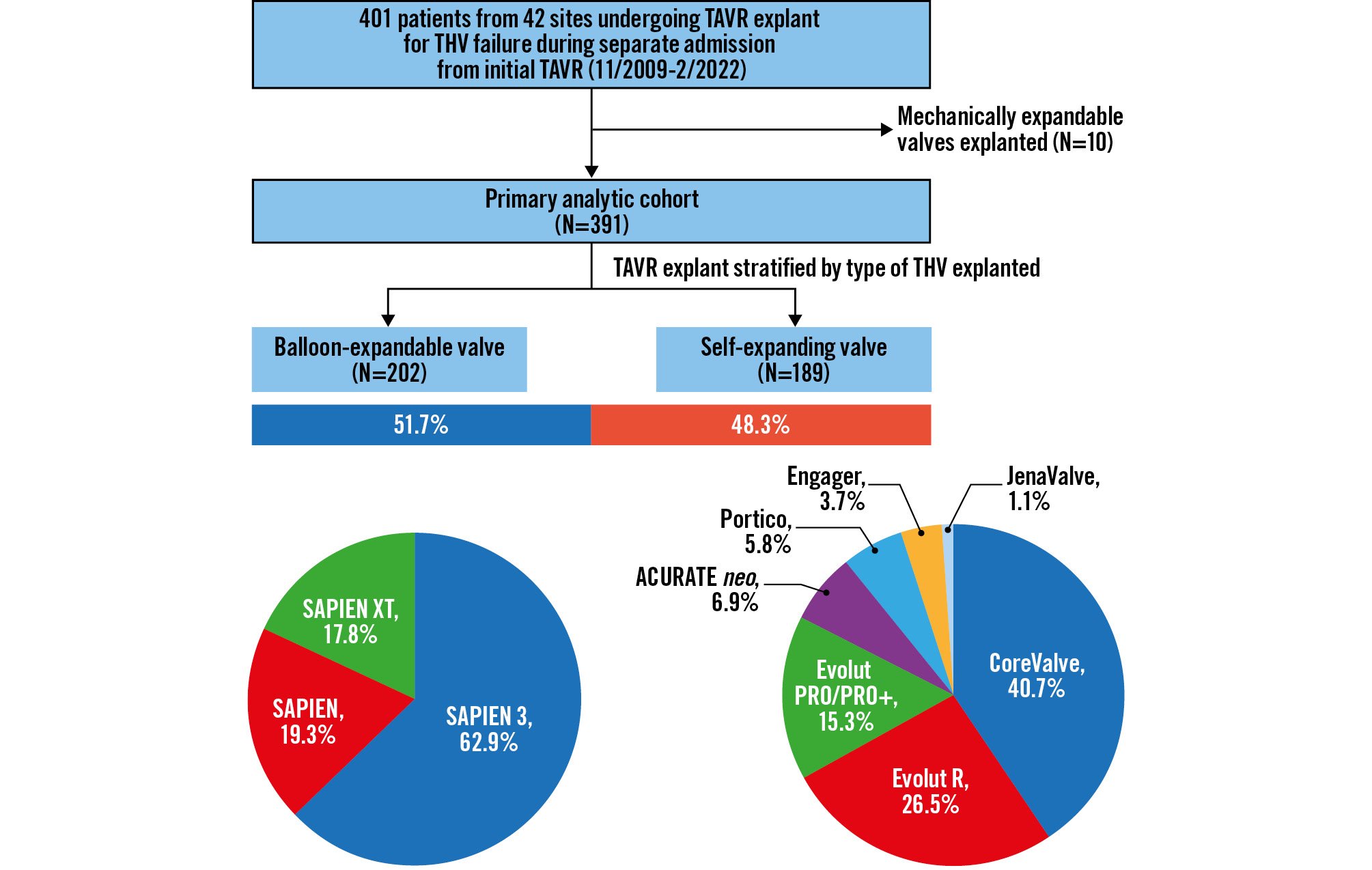
Figure 1. Study population. From November 2009 to February 2022, 401 patients from 42 sites in the international EXPLANT-TAVR registry underwent TAVR explant for transcatheter valve failure during a separate admission from the initial TAVR. All TAVR explants for mechanically expandable valves (N=10, 2.5%) were excluded. Outcomes of 202 (51.7%) BEV were compared with 189 (48.3%) SEV. BEV: balloon-expandable valve; SEV: self-expanding valve; TAVR: transcatheter aortic valve replacement; THV: transcatheter heart valve
OUTCOMES OF INTEREST AND DEFINITIONS
The primary outcomes of interest were intraoperative, in-hospital, 30-day, and 1-year mortality, and cumulative mortality at 3 years. The secondary outcomes of interest included the median interval from the index TAVR procedure to TAVR explant, in-hospital rates of complications, the median intensive care unit (ICU) and hospital length of stay, 30-day readmission rates and stroke rates at 30 days and 1 year. All indications for TAVR explant and clinical endpoints, including SVD, and PVL severity, were reported according to the Valve Academic Research Consortium-3 criteria8. The timing of TAVR explant was classified, based on the time interval between the diagnosis of needing surgery and undergoing the surgical explantation, as previously described7. The interval from index TAVR to TAVR explant was calculated, in months, as the time between the dates of the two procedures. Survival was reported in months from the date of TAVR explant to mortality date or the date of last follow-up if the patient was still alive.
STATISTICAL ANALYSIS
Continuous variables are reported as means with standard deviation or median with interquartile range (IQR), depending on the distribution of data. Normal distribution was examined using the Kolmogorov-Smirnov test. Categorical variables are reported as percentages. Depending on the distribution of data, differences between the failed BEV and failed SEV groups were detected using the Student’s 2-sample t-test or Mann-Whitney U test for the continuous variables and the chi-squared or Fisher’s exact test for the categorical variables. Kaplan-Meier survival analysis was used to assess actuarial freedom from all-cause mortality, separately for the overall cohort, and stratified by THV type at TAVR explant.
An exploratory analysis to identify independent predictors of all-cause mortality after TAVR explant within each THV group was performed. Since model building was limited by the relatively low number of mortality events, only forward, stepwise, multivariable Cox regression models were developed. All variables with p<0.10 from univariable analysis, in addition to clinically relevant variables chosen a priori (including age and STS-Predicted Risk of Mortality [PROM] at index TAVR) and deemed to influence the outcomes of interest, were considered in multivariable Cox regression analysis, and only those with p<0.05 were included in the final model. Subgroup analysis was performed to determine the impact of THV type on all-cause mortality in various prespecified subgroups of interest. All statistical tests were 2-tailed, with p<0.05 considered significant. Statistical analyses were performed using SPSS version 24.0 (IBM).
Results
BASELINE CLINICAL CHARACTERISTICS AT INDEX TAVR
A total of 391 patients underwent TAVR explant for failed BEV (N=202, 51.7%) or failed SEV (N=189, 48.3%), as per the inclusion criteria. Baseline clinical characteristics are summarised in Table 1. The mean age was 73.0±9.8 years, and 33.8% were women, with no differences between groups. There were also no differences in the prevalence of comorbidities between the two groups, with the exception of more hostile mediastinum (10.9% vs 4.1%; p=0.017) and previous cardiac surgery (45.7% vs 31.7%; p=0.006) in the failed-SEV group. At index TAVR, 24.1% of patients were deemed low surgical risk by the local Heart Team. While there were no differences in surgical risk at index TAVR or TAVR explant between the two groups, the median STS risk score for SAVR increased significantly from the time of index TAVR to TAVR explant in both the failed-BEV (2.8% [IQR 1.9-5.0] to 5.1% [IQR 2.9-8.9]; p<0.001) and failed-SEV (3.3% [IQR 2.2-5.5] to 5.0% [IQR 3.0-8.5]; p<0.001) groups. The temporal trends of annual TAVR explant from 2009 to 2022 for failed BEV and failed SEV are illustrated in Supplementary Figure 1.
Table 1. Patient characteristics at the time of index TAVR.
| Variables | BEV (N=202) | SEV (N=189) | p-value |
|---|---|---|---|
| Age, years | 73.4 [9.2] | 72.1 [10.3] | 0.21 |
| Female | 68 (33.7) | 64 (33.9) | 1.00 |
| Frailty | 69 (36.3) | 54 (31.6) | 0.37 |
| Coronary artery disease | 115 (58.4) | 98 (53.0) | 0.30 |
| Stroke | 36 (18.3) | 28 (15.1) | 0.41 |
| Cerebrovascular disease | 64 (32.5) | 45 (24.2) | 0.089 |
| Peripheral vascular disease | 47 (23.9) | 31 (16.7) | 0.099 |
| Diabetes | 69 (35) | 70 (37.4) | 0.67 |
| Atrial fibrillation | 77 (39.1) | 84 (44.9) | 0.26 |
| Pulmonary hypertension | 57 (29.7) | 55 (30.6) | 0.91 |
| Chronic kidney disease | 77 (40.1) | 74 (40.4) | 1.00 |
| Dialysis-dependent | 9 (4.6) | 10 (5.4) | 0.82 |
| Chronic obstructive pulmonary disease | 59 (29.9) | 47 (25.3) | 0.36 |
| Hostile chest or chest deformity | 8 (4.1) | 20 (10.9) | 0.017 |
| Porcelain aorta | 13 (6.7) | 8 (4.4) | 0.38 |
| Left ventricular ejection fraction, % | 52.3 [12.4] | 50.9 [13.4] | 0.29 |
| Prior permanent pacemaker/ICD | 40 (20.2) | 47 (25.3) | 0.27 |
| Prior percutaneous coronary intervention | 60 (30.2) | 48 (25.8) | 0.37 |
| Body surface area, m² | 2 [0.3] | 2 [0.3] | 0.10 |
| New York Heart Association Class | |||
| 1 | 10 (5.7) | 11 (6.4) | 0.83 |
| 2 | 50 (28.7) | 43 (24.9) | 0.47 |
| 3 | 92 (52.9) | 85 (49.1) | 0.52 |
| 4 | 22 (12.6) | 34 (19.7) | 0.082 |
| Previous cardiac surgery | 63 (31.7) | 85 (45.7) | 0.006 |
| Society of Thoracic Surgeons Predicted Risk of Mortality, % | 2.8 | 3.3 | 0.10 |
| [1.9-5.0] | [2.2-5.5] | ||
| EuroSCORE II | 4.7 | 5.1 | 0.24 |
| [2.7-9.2] | [2.6-10.0] | ||
| Heart Team risk stratification | |||
| Low | 32 (23.7) | 36 (24.5) | 0.89 |
| Intermediate | 60 (44.4) | 52 (35.4) | 0.14 |
| High | 36 (26.7) | 49 (33.3) | 0.24 |
| Extreme | 7 (5.2) | 10 (6.8) | 0.62 |
| All variables are expressed as mean [standard deviation], median [interquartile range] or N (%). p-values in red: p<0.05; p-values in blue: p>0.05 & <0.10 (to indicate trend). BEV: balloon-expandable valve; EuroSCORE: European System for Cardiac Operative Risk Evaluation; ICD: implantable cardioverter defibrillator; SEV: self-expanding valve; TAVR: transcatheter aortic valve replacement | |||
PROCEDURAL CHARACTERISTICS AT TAVR EXPLANT
The most frequent indications for TAVR explant were endocarditis (36.0% failed SEV vs 55.4% failed BEV; p<0.001), SVD (30.2% vs 21.8%; p=0.065), PVL (21.2% vs 11.9%; p=0.014) and PPM (8.5% vs 10.4%; p=0.61) (Table 2, Supplementary Figure 2). After excluding the endocarditis cohort, there were no differences in the primary reasons for exclusion from redo-TAVR between groups; these included unfavourable anatomy for redo-TAVR (20.9% in BEV vs 19.5% in SEV; p=0.73) and prior valve-in-valve replacement (7.1% vs 8.5%; p=0.58). The median time from index TAVR to TAVR explant was 12.7 months (IQR 5.4-28.9) after BEV TAVR and 15.0 months (IQR 4.0-38.5) after SEV TAVR, with no differences between groups overall (p=0.63) or when stratified by indication for surgery (Figure 2). The most common THV device explanted within each group was the SAPIEN 3 THV (Edwards Lifesciences; 62.9%) in the BEV group and the CoreValve (Medtronic; 40.7%) in the SEV group.
Emergency and urgent cases compromised 3.8% and 53.6% of all cases, respectively, with the SEV group having fewer urgent/emergency cases than the BEV group (52.0% vs 62.3%; p=0.057). Aortic root replacement was more frequently performed in the SEV group (15.3% vs 7.4%; p=0.016). Surgical bioprostheses were implanted in 85.4%
Table 2. Procedural characteristics in patients who underwent TAVR explant.
| Variables | BEV (N=202) | SEV (N=189) | p-value |
|---|---|---|---|
| Primary clinical indications for AVR | |||
|
Prosthetic valve endocarditis |
112 (55.4) | 68 (36) | <0.001 |
|
Structural valve deterioration |
44 (21.8) | 57 (30.2) | 0.065 |
|
Paravalvular leak |
24 (11.9) | 40 (21.2) | 0.014 |
|
Prosthesis-patient mismatch |
21 (10.4) | 16 (8.5) | 0.61 |
|
Prosthetic valve migration |
4 (2) | 6 (3.2) | 0.53 |
|
Other |
13 (6.4) | 19 (10.1) | 0.20 |
| Society of Thoracic Surgeons Predicted Risk of Mortality, % | 5.1 | 5.0 | 0.52 |
| [2.9-8.9] | [3.0-8.5] | ||
| Timing of operation | |||
|
Elective |
73 (37.8) | 83 (48) | 0.057 |
|
Urgent |
111 (57.5) | 85 (49.1) | 0.12 |
|
Emergent |
9 (4.7) | 5 (2.9) | 0.43 |
| Explanted valve size, mm | 26 | 29 | |
| [23-26] | [26-29] | ||
| Time from TAVR to explant, months | 12.7 | 15.0 | 0.27 |
| [5.4-28.9] | [4.0-38.5] | ||
| Cardiopulmonary bypass time, mins | 129 | 141 | 0.54 |
| [103-174] | [100-194] | ||
| Aortic cross-clamp time, mins | 95 | 97 | 0.57 |
| [73-127] | [68-153] | ||
| Implanted valve size, mm | 23 | 23 | |
| [21-25] | [23-25] | ||
| Aortic valve replacement | 187 (92.6) | 160 (84.7) | 0.016 |
|
Mechanical |
28 (15.0) | 25 (15.6) | 0.88 |
|
Tissue |
159 (85.0) | 135 (84.4) | |
| Root replacement | 15 (7.4) | 29 (15.3) | 0.016 |
|
Mechanical |
2 (13.3) | 2 (6.9) | 0.60 |
|
Tissue |
13 (86.7) | 27 (93.1) | |
| Concomitant procedure(s) | 117 (57.9) | 109 (57.7) | 1.00 |
|
Ascending aortic replacement |
9 (7.7) | 10 (9.2) | 0.81 |
|
Coronary artery bypass graft |
33 (28.2) | 23 (21.1) | 0.22 |
|
Root repair |
7 (6) | 4 (3.7) | 0.54 |
|
Mitral valve surgery |
42 (35.9) | 46 (42.2) | 0.34 |
|
Tricuspid valve surgery |
11 (9.4) | 22 (20.2) | 0.024 |
|
Mitral or tricuspid valve surgery |
47 (40.2) | 54 (49.5) | 0.18 |
| Root enlargement | 22 (10.9) | 26 (13.8) | 0.44 |
| All variables are expressed as mean [standard deviation], median [interquartile range] or N (%). p-values in red: p<0.05; p-values in blue: p>0.05 & <0.10 (to indicate trend). AVR: aortic valve replacement; BEV: balloon-expandable valve; SEV: self-expanding valve; TAVR: transcatheter aortic valve replacement | |||
of cases, with no differences between groups. The median cardiopulmonary bypass time (132 minutes [IQR 103-186]) and aortic cross-clamp time (95 minutes [IQR 70-136]) were also similar between groups. Among 57.8% of patients undergoing concomitant cardiac procedures during TAVR explant, CABG (24.8%), and mitral (38.9%) and tricuspid (14.6%) valve surgery were the most frequent concurrent procedures performed. There were no differences in concomitant non-aortic procedures between groups, with the exception of more frequent tricuspid valve surgery during SEV explantation (20.2% vs 9.4%; p=0.024).
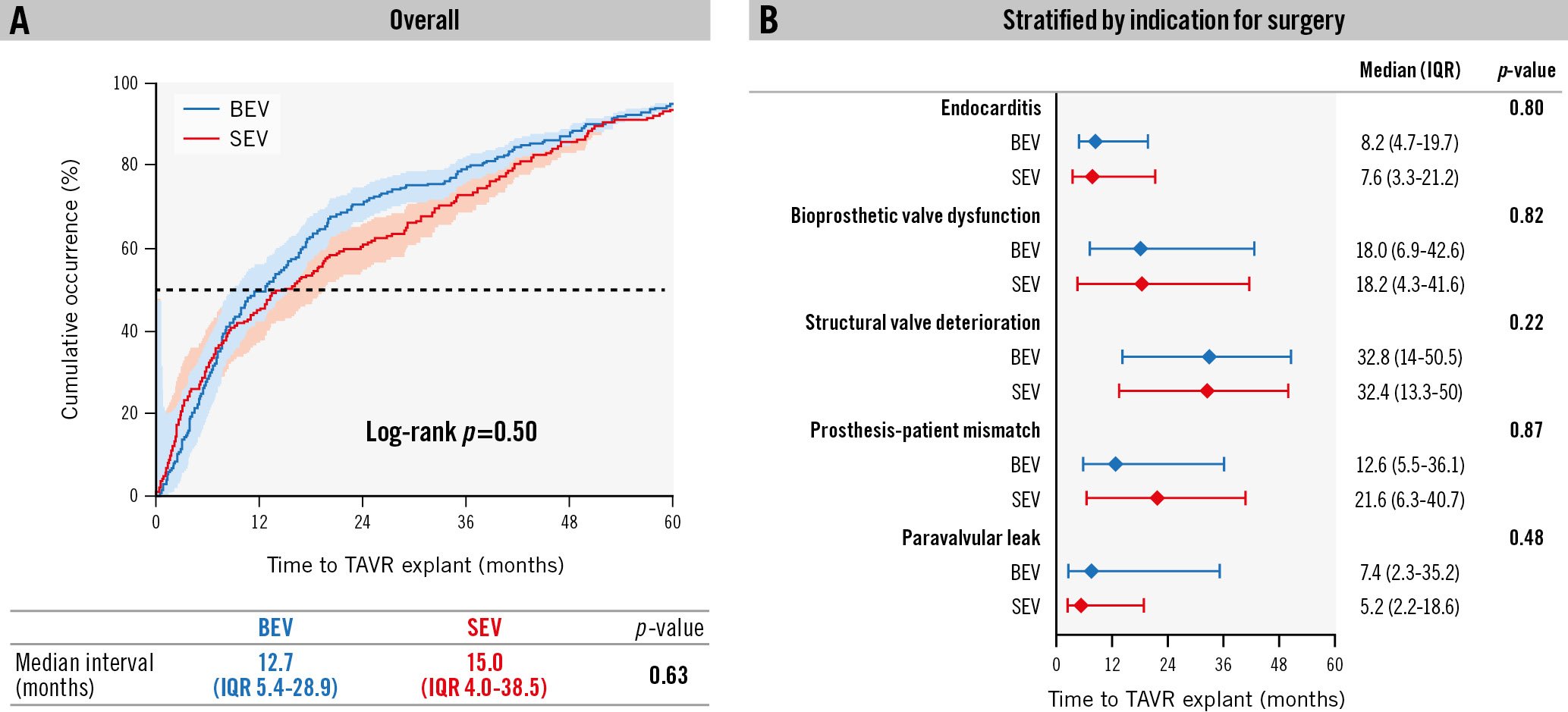
Figure 2. Timing of TAVR explant. There were no significant differences in the median interval from index TAVR to TAVR explant between the BEV and SEV groups overall (A) or by indication for TAVR explant (B). BEV: balloon-expandable valve; IQR: interquartile range; SEV: self-expanding valve; TAVR: transcatheter aortic valve replacement
POSTPROCEDURAL AND MIDTERM CLINICAL OUTCOMES
The overall rates of intraoperative and in-hospital mortalities were 0.8% and 13.0%, respectively, with no significant differences between groups. There were also no differences between the two groups in the duration of mechanical ventilation, ICU stay, hospital stay, new pacemaker implantation, in-hospital stroke, vascular complications, or major or life-threatening bleeding events (Table 3). At 30 days, there were no significant differences in mortality (15.1% vs 17.3%; p=0.57), stroke (4.4% vs 7.1%; p=0.36) or readmission rates (13.9% vs 8.9%; p=0.17) between the BEV and SEV groups, respectively; both groups demonstrated similar mean aortic valve gradients (12.3±11.7 vs 9.9±4.4 mmHg; p=0.11). Among 247 patients who completed 1-year follow-up, mortality was 32.8%, with no differences between groups (31.8% BEV vs 33.9% SEV). The overall median follow-up (including all mortality) was 30.4 months (IQR 14.4-51.1) from the index TAVR and 6.6 months (IQR 1.0-18.8) after TAVR explant. There were no differences in actuarial estimates of cumulative mortality at 3 years between groups (log-rank p=0.95) (Central illustration).
Table 3. Outcomes after TAVR explant.
| Variables | BEV (N=202) | SEV (N=189) | p-value |
|---|---|---|---|
| Intraoperative mortality | 2 (1) | 1 (0.5) | 1.00 |
| In-hospital mortality | 24 (11.9) | 27 (14.3) | 0.55 |
| Ventilator hours | 16 | 19 | 0.70 |
| [8-37] | [10-37] | ||
| ICU length of stay, hours | 75 | 72 | 0.75 |
| [38-168] | [32-161] | ||
| Hospital length of stay, days | 12 | 13 | 0.50 |
| [8-21] | [8-19] | ||
| New permanent pacemaker* | 29/158 (18.4) | 24/137 (17.5) | 0.88 |
| In-hospital stroke | 7 (3.6) | 11 (6) | 0.34 |
| In-hospital vascular complication | 9 (4.6) | 2 (1.1) | 0.064 |
| In-hospital life-threatening bleed | 14 (7.1) | 11 (6) | 0.84 |
| In-hospital major bleed | 23 (11.7) | 24 (13.2) | 0.76 |
| 30-day | |||
| Mortality | 28/186 (15.1) | 30/173 (17.3) | 0.57 |
| Stroke | 8 (4.4) | 12 (7.1) | 0.36 |
| Readmission | 23 (13.9) | 14 (8.9) | 0.17 |
| Mean aortic gradient, mmHg | 12.3 [11.7] | 9.9 [4.4] | 0.11 |
| 1-year | |||
| Mortality | 42/132 (31.8) | 39/115 (33.9) | 0.79 |
| Stroke | 11 (8.3) | 15 (12.8) | 0.30 |
| All variables are expressed as mean [standard deviation], median [interquartile range], N (%) or n/N (%). p-values in blue: p>0.05 & <0.10 (to indicate trend). *Patients with prior pacemaker or implantable cardioverter defibrillator were excluded. BEV: balloon-expandable valve; ICU: intensive care unit; SEV: self-expanding valve; TAVR: transcatheter aortic valve replacement | |||
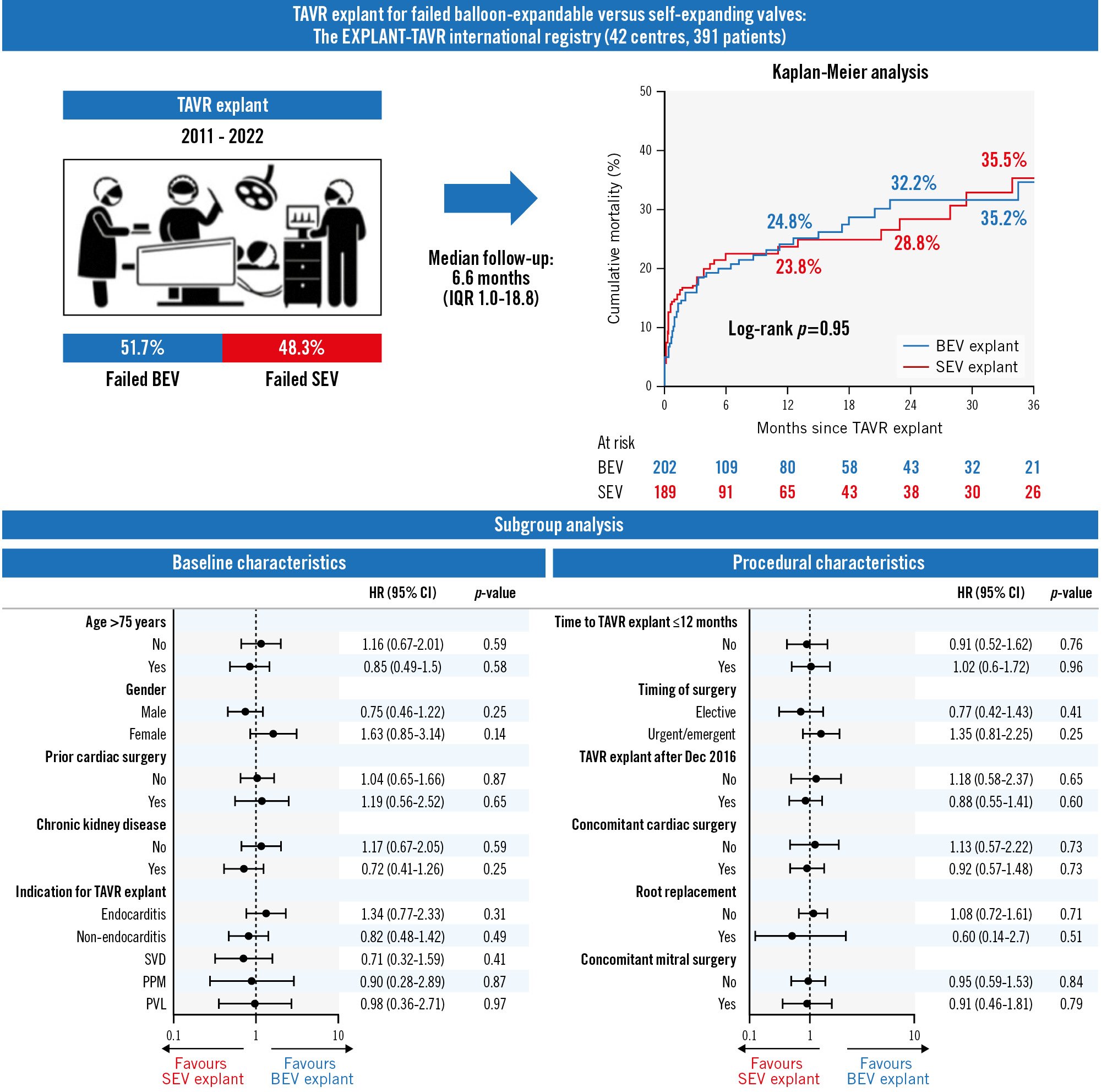
Central illustration. Impact of THV type on mortality after TAVR explant. Kaplan-Meier analysis showed no differences in actuarial estimates of 3-year cumulative mortality between groups (35.2% BEV vs 35.5% SEV; log-rank p=0.95) (A). THV type also had no significant impact on mortality after TAVR explant in various prespecified subgroups of interest (B). BEV: balloon-expandable valve; CI: confidence interval; HR: hazard ratio; IQR: interquartile range; PPM: prosthesis-patient mismatch; PVL: paravalvular leak; SEV: self-expanding valve; SVD: structural valve deterioration; TAVR: transcatheter aortic valve replacement; THV: transcatheter heart valve
PREDICTORS OF ALL-CAUSE MORTALITY AFTER TAVR EXPLANT
On univariate analysis, chronic kidney disease (CKD), New York Heart Association (NYHA) Class, longer cardiopulmonary bypass (CPB) and cross-clamp times were associated with mortality after BEV explant (Figure 3A). After multivariable logistic regression, peripheral vascular disease (hazard ratio [HR] 1.95, 95% confidence interval [CI]:1.08-3.50), dialysis (HR 4.68, 95% CI:1.95-11.25), emergency surgery (HR 4.66, 95% CI: 1.94-11.22) and concomitant mitral surgery (HR 2.00, 95% CI: 1.07-3.72) were found to be independent predictors of all-cause mortality. Similarly, pulmonary hypertension was associated with mortality after SEV explant, while diabetes (HR 1.94, 95% CI: 1.07-3.54), cirrhosis (HR 2.42, 95% CI: 1.07-5.50), longer CPB time (HR 1.22 per hour, 95% CI: 1.001-1.49) and concomitant mitral surgery (HR 2.00, 95% CI: 1.08-3.69) were independent predictors of all-cause mortality (Figure 3B).
Neither surgical risk, determined by the STS risk score or the local Heart Team during index TAVR, nor indication for TAVR explant were associated with mortality after TAVR explant in either the BEV or SEV group. After adjusting for baseline differences in a multivariable Cox regression model with THV type as a covariate, the THV type had no significant impact on all-cause mortality after TAVR explant (unadjusted HR 0.99, 95% CI: 0.67-1.45; adjusted HR 1.15, 95% CI: 0.76-1.75) (Supplementary Table 1). THV type also had no significant impact on mortality after TAVR explant in subgroup analyses stratifying patients based on various prespecified cohorts of interest, including age >75 years, sex, prior cardiac surgery, CKD, time to TAVR explant, TAVR explant generation, indication and timing of surgery, in addition to concomitant cardiac/mitral surgery and root replacement during TAVR explant (all p<0.05) (Central illustration). Cumulative mortality at 2 years was similar between the BEV and SEV groups (32.7% vs 28.4% respectively; p=0.49) even after excluding all TAVR explants for endocarditis (Supplementary Figure 3).
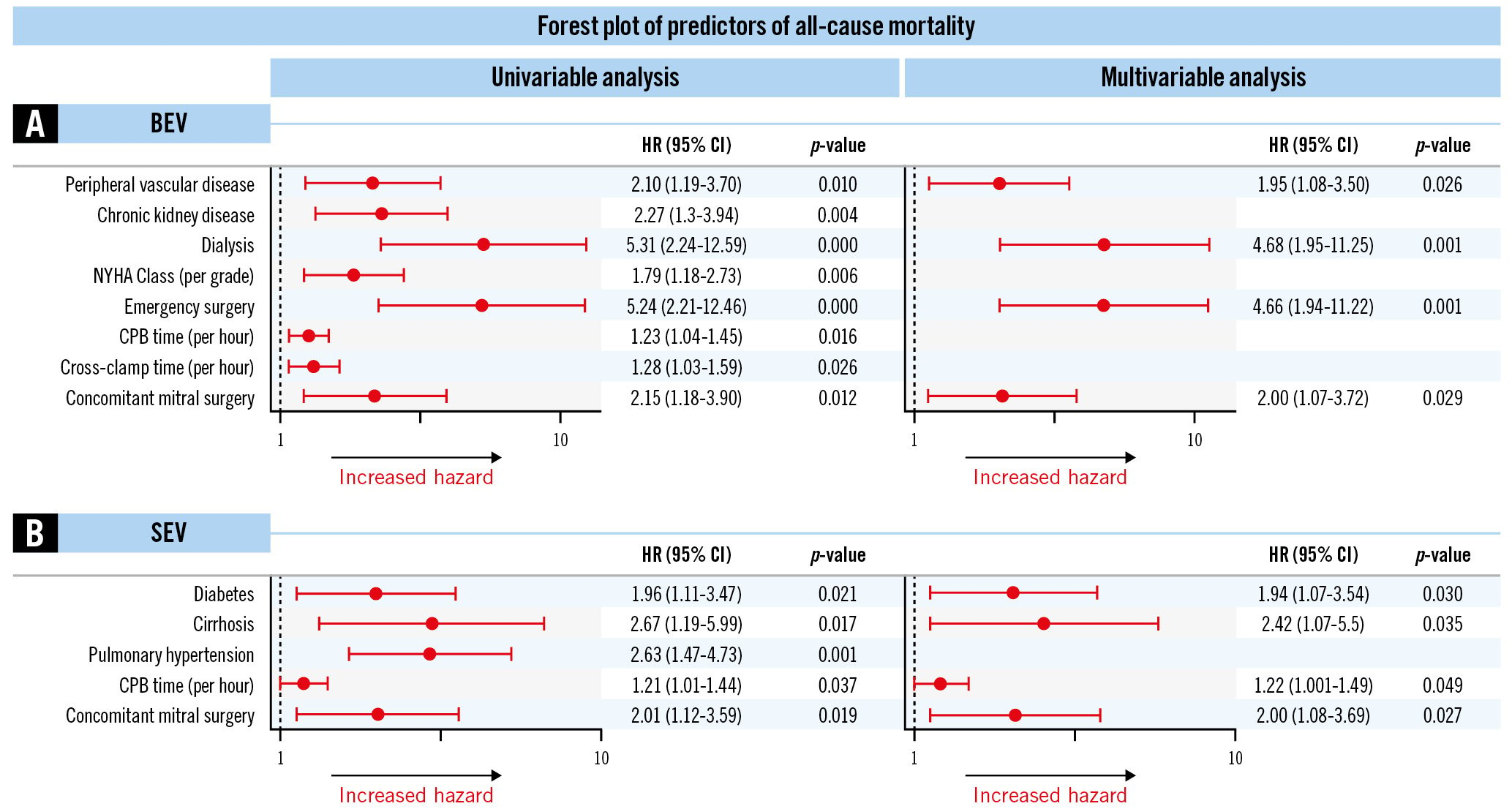
Figure 3. Predictors of all-cause mortality after TAVR explant. Forest plot showing univariate and multivariable predictors of 1-year mortality after BEV explant (A) and SEV explant (B). BEV: balloon-expandable valve; CI: confidence interval; CPB: cardiopulmonary bypass; HR: hazard ratio; NYHA: New York Heart Association; SEV: self-expanding valve; TAVR: transcatheter aortic valve replacement
Discussion
This study, a subgroup analysis of the international EXPLANT-TAVR registry, compared the characteristics and outcomes of patients undergoing TAVR explant for failed BEV versus failed SEV. Our key findings are as follows: 1) The failed SEV group had fewer cases of endocarditis and more PVL as primary indications for TAVR explant, with no differences in SVD or PPM between groups. 2) SEV explant was associated with more frequent aortic root replacement and trended fewer urgent/emergency cases. 3) Mortality after TAVR explant was high (16% at 30 days; 33% at 1 year) but was not associated with the type of THV explanted, despite adjusting for baseline differences and in subgroup analyses. 4) In a significant proportion of TAVR explants requiring concomitant cardiac procedures (58%), mitral surgery was the most common combined procedure and was an independent predictor of mortality in both groups.
DIFFERENT INDICATIONS FOR TAVR EXPLANT IN PATIENTS WITH FAILED BEV VERSUS FAILED SEV
Endocarditis (46%) was the predominant mode of THV failure in our study, followed by SVD (26%) and significant PVL (16%). Prosthetic valve endocarditis is associated with significant mortality and morbidity, and has a reported incidence of 1.0%/person-year after TAVR9. While TAVR explant remains the mainstay of definitive therapy, redo-TAVR may be considered for treating sequelae such as severe aortic regurgitation after completion of antibiotic treatment and clearance of infection in select patients with no surgical option. However, the impact of THV device type on the development of endocarditis remains unclear, with conflicting data regarding differences in incidence between THV types. For example, while there were no differences in the 1-year frequency (1.25% vs 0.95%; p=0.33) or TAVR explant rates (13.8% vs 8.7%; p=0.21) between the BEV and SEV groups, respectively, in the Infectious Endocarditis After TAVR Registry10, another study reporting outcomes after TAVR explant using the STS national database showed that patients with BEV prostheses more frequently had endocarditis than those with SEV (24% vs 13%; p=0.006)11. In our study, while the failed BEV group had more endocarditis, the failed SEV group had more PVL as the indication for TAVR explant, with no differences in SVD or PPM between groups. SEV patients in our study had a greater degree of PVL (>mild PVL: 43.0% SEV vs 23.5% BEV; p<0.001) after the index TAVR procedure, which is in line with a meta-analysis showing more significant PVL after SEV TAVR12. Furthermore, significant PVL may not be amenable to percutaneous closure, especially in SEV patients, given the difficulty in traversing the stent frame13. TAVR explant may be the only treatment option in these cases.
MORE AORTIC ROOT REPLACEMENT WITH FAILED SEV
THV design can present technical challenges during TAVR explant, as THV interaction with the surrounding structures is different for SEV, which are taller than BEV. A higher aortotomy may be necessary to extract the taller stent frame of SEV, where visualisation of the aortic annular complex is often challenging and can result in more root damage. On the other hand, surgical explantation of shorter BEV may be more familiar to the non-TAVR surgeon, as their stent profile is similar to surgical aortic valves. This may explain why aortic root replacements are more frequently performed during SEV explantation compared to BEV (15.3% vs 7.4%; p=0.016). However, there were no differences in ascending aortic replacement between THV groups. These findings are contrary to prior reports from the STS database, where Fukuhara et al reported similar rates of root replacement between THV groups (18.9% BEV vs 22.1% SEV; p=0.52) but higher rates of ascending aortic replacement with SEV (18.2% vs 8.2%; p=0.009)14. Patients in both studies, however, had similarly high 30-day mortality.
CONCOMITANT CARDIAC SURGERY IN TAVR EXPLANT WAS COMMON FOR BOTH THV TYPES
Patients undergoing TAVR often have concomitant valvular or coronary disease that may progress despite successful TAVR, and they may not be candidates for additional transcatheter therapies. When such patients undergo cardiac surgery, TAVR explant may be necessary if THV compression/deformity occurs. Conversely, any inadvertent injury to the surrounding structures during TAVR explant may warrant a more complex procedure, not just an isolated SAVR. Deep valve implantation, particularly with earlier-generation THVs, may impinge on the aortomitral curtain and anterior mitral leaflet as well as the membranous septum. If these structures are injured during TAVR explant, repair of the ventricular septal defect or concomitant mitral surgery will be required. Although we are unable to differentiate between valvular disease progression versus iatrogenic injury during TAVR explant as the primary indication for concomitant surgery, the burden of concomitant cardiac surgery during TAVR explant was significant (at nearly 60%) and similar to prior reported studies1114. In our series, concomitant mitral (40%), coronary artery bypass (25%), and tricuspid valve surgery (15%) were the most frequent concurrent procedures performed. Interestingly, similar to the STS report by Fukuhara et al11, there were no differences in concomitant non-aortic procedures performed between the BEV and SEV groups in our study, with the exception of more frequent tricuspid valve surgery during SEV explantation (20.2% vs 9.4%; p=0.024).
Mitral valve surgery was the most frequently performed concomitant procedure in our study and an independent predictor of mortality in both BEV and SEV groups. Although we could not determine from our registry whether patients undergoing concomitant mitral surgery met criteria for intervention at the time of the index TAVR procedure, these patients likely represented the highest-risk subgroup in our study, as evidenced by higher surgical risk at index TAVR and a more complex double valve operation with longer cross-clamp and CPB times at TAVR explant regardless of the THV type, which may explain the worse outcomes in this cohort. The elevated mortality and morbidity rates may simply reflect early surgical experience.
MORTALITY AFTER TAVR EXPLANT REMAINED HIGH IRRESPECTIVE OF THV TYPE
There is growing evidence suggesting that the mortality and morbidity associated with TAVR explant are not negligible; with an increased observed-to-expected mortality5610. The results from our international multicentre registry further shed light on the impact of THV type on outcomes after TAVR explant. We found that mortality after TAVR explant was high (16% at 30 days; 33% at 1 year) but was not associated with the type of THV explanted, despite adjusting for baseline differences and performing subgroup analysis. Our findings are consistent with those reported by Fukuhara et al from the STS national database11 and are not surprising considering there were no differences between the two groups in terms of surgical risk at index TAVR or subsequent TAVR explant procedure, with similar CPB and cross-clamp times. There were also no significant differences in postoperative or midterm outcomes between the BEV and SEV groups despite more frequent root replacement in patients with failed SEV. One potential explanation is that the threshold for performing aortic root replacement may be higher in patients with comorbidities, as shown in our prior subgroup analysis between patients with SAVR and root replacement after TAVR explant15. Nonetheless, our findings suggest that while THV design may pose technical challenges during TAVR explant, it does not significantly influence outcomes after surgery.
Limitations
Despite the strengths of our multicentre international registry-based study, it is a retrospective observational analysis with all the inherent limitations. First, we are limited by our overall sample size and the relatively low number of mortality events in demonstrating statistically significant differences in mortality between groups, and only forward, stepwise, multivariable Cox regression models were developed. Second, the retrospective nature of this study and the long study period may have introduced time selection and learning curve biases. Third, the primary indication for TAVR explant and reasons for exclusion from redo-TAVR were assessed independently by the respective Heart Teams at each institution, which may have introduced patient selection biases. We were unable to account for qualifying patients that did not undergo or declined TAVR explant. Fourth, the volume of TAVR procedures performed outside participating centres that were referred to our participating sites for reintervention was not captured. Finally, we were unable to account for the potential impact of procedural volume and operator/centre-level variations in transcatheter and surgical techniques on clinical outcomes. The decision to perform root replacement and/or additional cardiac procedures was at the surgeon’s discretion at the time of procedure.
Conclusions
In the EXPLANT-TAVR global registry, BEV and SEV groups had different indications for surgical explantation with more ascending/root replacements in patients with SEV failure, but no differences in short- and midterm mortality and morbidity. Further refinement of TAVR explant techniques will be important for the improvement of outcomes.
Impact on daily practice
In light of the significant mortality and morbidity associated with TAVR explant for THV failure, the clinical impact of THV type (BEV vs SEV) after TAVR explant remains unknown. In our study using the EXPLANT-TAVR global registry, compared to patients with failed BEV, those with failed SEV had fewer cases of endocarditis and more PVL as primary indications for TAVR explant, with no differences in SVD and PPM between groups. Mortality after TAVR explant was high (16% at 30 days; 33% at 1 year) but was not associated with the type of THV explanted after adjusting for baseline differences and performing subgroup analysis, despite more frequent aortic root replacement and fewer urgent/emergency cases in SEV explant.
Appendix. Study collaborators.
Giuseppe Bruschi, MD; ASST Niguarda General Hospital, Milan, Italy; Katherine B. Harrington, MD; Baylor, Scott & White The Heart Hospital, Plano, TX, USA; John J. Squiers, MD; Baylor, Scott & White The Heart Hospital, Plano, TX, USA; J. Michael DiMaio, MD; Baylor, Scott & White The Heart Hospital, Plano, TX, USA; Sameer Hirji, MD, MPH; Brigham & Women's Hospital, Boston, MA, USA; Pinak B. Shah, MD; Brigham & Women's Hospital, Boston, MA, USA; Flavien Vincent, MD; CHU Lille, University of Lille, Lille, France; Mohammad Koussa, MD; CHU Lille, University of Lille, Lille, France; Isaac George, MD; Columbia University Irving Medical Center, New York, NY, USA; Nicholas M. Van Mieghem, MD, PhD; Erasmus University Medical Center, Rotterdam, the Netherlands; Thijmen W. Hokken, MD; Erasmus University Medical Center, Rotterdam, the Netherlands; Joerg Kempfert, MD; German Heart Center Berlin, Berlin, Germany; Keti Vitanova, MD; German Heart Center Munich, Munich, Germany; Rudiger Lange, MD; German Heart Center Munich, Munich, Germany; Marvin D. Atkins, MD; Houston Methodist DeBakey Heart and Vascular Center, Houston, TX, USA; John R. Doty, MD; Intermountain Health, Salt Lake City, UT, USA; Brian K. Whisenant, MD; Intermountain Health, Salt Lake City, UT, USA; Andrea Garatti, MD; IRCCS Policlinico San Donato, Milan, Italy; Marco Di Eusanio, MD; Lancisi Cardiovascular Center, OORR, Politechnic University of Marche, Ancona, Italy; Filippo Capestro, MD; Lancisi Cardiovascular Center, OORR, Politechnic University of Marche, Ancona, Italy; Philipp Kiefer, MD; Leipzig Heart Center, Leipzig, Germany; David Holzhey, MD; Leipzig Heart Center, Leipzig, Germany; Thilo Noack, MD; Leipzig Heart Center, Leipzig, Germany; Michael A. Borger, MD; Leipzig Heart Center, Leipzig, Germany; Luigi Pirelli, MD; Lenox Hill Hospital, Northwell Health, New York, NY, USA; Shekhar Saha, MD; LMU Klinikum Munich, Munich, Germany; Christian Hagl, MD; LMU Klinikum Munich, Munich, Germany; Muhanad Algadheeb, MD; London Health Sciences Center, Western University, London, ON, Canada; Michael W. A. Chu, MD; London Health Sciences Center, Western University, London, ON, Canada; Antonio Di Virgilio, MD; Mater Domini University Hospital, Catanzaro, Italy; Michael H. Salinger, MD; Medical College of Wisconsin, Milwaukee, WI, USA; Paul Werner, MD; Medical University of Vienna, Vienna, Austria; Christian Shults, MD; MedStar Washington Hospital Center, Washington, D.C., USA; Lowell F. Satler, MD; MedStar Washington Hospital Center, Washington, D.C., USA; Walid Ben Ali, MD, PhD; Montreal Heart Institute, Montreal, QC, Canada; Philippe Demers, MD; Montreal Heart Institute, Montreal, QC, Canada; Reda Ibrahim, MD; Montreal Heart Institute, Montreal, QC, Canada; Joshua Rovin, MD; Morton Plant Hospital, Clearwater, FL, USA; Pierre Voisine, MD; Quebec Heart and Lung Institute, Laval University, Quebec City, QC, Canada; Josep Rodés-Cabau, MD, PhD; Quebec Heart and Lung Institute, Laval University, Quebec City, QC, Canada; Guido Gelpi, MD; Sacco Hospital, ASST-Fatebenefratelli-Sacco, University of Milan, Milan, Italy; Igor Belluschi, MD; San Raffaele University Hospital, Milan, Italy; Francesco Maisano, MD; San Raffaele University Hospital, Milan, Italy; George A. Petrossian, MD; St. Francis Hospital, Roslyn, NY, USA; Maral Ouzounian, MD; University Health Network, Toronto, Toronto, ON, Canada; Oliver D. Bhadra, MD; University Heart and Vascular Center Hamburg, Hamburg, Germany; Rodrigo Estevez-Loureiro, MD, PhD; University Hospital Alvaro Cunqueiro, Vigo, Spain; Miguel A. Pinon, MD; University Hospital Alvaro Cunqueiro, Vigo, Spain; Moritz Wyler von Ballmoos, MD, PhD, MPH; University Hospital Zurich, Zurich, Switzerland; Shinichi Fukuhara, MD; University of Michigan, Ann Arbor, MI, USA; G. Michael Deeb, MD; University of Michigan, Ann Arbor, MI, USA; Marc Ruel, MD; University of Ottawa Heart Institute, Ottawa, ON, Canada; Talal Al-Atassi, MD; University of Ottawa Heart Institute, Ottawa, ON, Canada; Augusto D'Onofrio, MD, PhD; University of Padova, Padova, Italy; Chiara Tessari, MD; University of Padova, Padova, Italy; Joseph E. Bavaria, MD; University of Pennsylvania, Philadelphia, PA, USA; Andrea Colli, MD; University of Pisa, Pisa, Italy; Alejandro Pizano, MD; University of Texas Health Science Center at Houston, Houston, TX, USA; Kashish Goel, MD; Vanderbilt University, Nashville, TN, USA; Ashish S. Shah, MD; Vanderbilt University, Nashville, TN, USA; Joshua B. Goldberg, MD; Westchester Medical Center, Valhalla, NY, USA; Arnar Geirsson, MD; Yale School of Medicine, New Haven, CT, USA; Maurizio Taramasso, MD, PhD; Zurich Heart Center, Zurich, Switzerland; Marco Gennari, MD; Zurich Heart Center, Zurich, Switzerland
Acknowledgements
We would like to thank all the co-investigators for their participation in the EXPLANT-TAVR registry.
Conflict of interest statement
G. Tang is a physician proctor and consultant for Medtronic; a consultant and physician advisory board member for Abbott Structural Heart; and a physician advisory board member for JenaValve. N.S. Kleiman has been involved in clinical trials for Edwards Lifesciences, Medtronic, and Boston Scientific; is involved in clinical education for Medtronic; and is on the steering committee for Boston Scientific. M. Szerlip is a physician proctor and consultant for Edwards Lifesciences; has received speaker honoraria for Boston Scientific; served as an advisory board member for Abbott; and is on the steering committee for Medtronic. M. Mack served as co-primary investigator for the PARTNER trial for Edwards Lifesciences and the COAPT trial for Abbott; and served as the study Chair for the APOLLO trial for Medtronic. T. Nazif has equity in Venus Medtech; and has received consulting fees or honoraria from Keystone Heart, Edwards Lifesciences, Medtronic, and Boston Scientific. A. Unbehaun has served as a physician proctor for Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. M. Andreas is a physician proctor and consultant and has received speaker honoraria from Edwards Lifesciences, Abbott, and Medtronic; and has received institutional research grants from Edwards Lifesciences, Abbott, Medtronic, and LSI Solutions. D. Brinster is a consultant and speaker for CryoLife, Cook Medical, and Terumo Aortic. B. Ramlawi is a consultant for Boston Scientific, Medtronic, LivaNova, and AtriCure. L. Conradi is a physician proctor, consultant and speaker for Edwards Lifesciences, Medtronic, Abbott, and Boston Scientific. N. Desai reports institution research funding and speaker fees from Gore and Medtronic. J. Forrest is a physician proctor, consultant, and member of the advisory board for Edwards Lifesciences and Medtronic. T. Nguyen has received speaker honoraria from Edwards Lifesciences, CryoLife, and Abbott. R. Waksman is a consultant and advisory board member for Abbott Vascular, Amgen, Boston Scientific, Medtronic, Philips/Volcano, and Pi-Cardia; has received speaker honoraria and grant support from AstraZeneca and Chiesi; has received grant support from Biotronik; is a consultant for Transmural Solutions; and is an investor for MedAlliance and Transmural Solutions. L. Leroux is a physician proctor for Medtronic and Abbott; and a consultant for Edwards Lifesciences. K. Grubb is a physician proctor for Medtronic, Edwards Lifesciences, and Boston Scientific; and has served as a consultant for Medtronic, Boston Scientific, Ancora, HLT, and BioVentrix. P. Denti receives speaker honoraria from Abbott and Edwards Lifesciences; and is a consultant for InnovHeart. T. Modine is a physician proctor and consultant for Medtronic, Edwards Lifesciences, and Abbott. V. Bapat has served as a consultant for Medtronic, Edwards Lifesciences, 4C Medical, and Boston Scientific. T. Kaneko is a speaker for Edwards Lifesciences, Medtronic, Abbott, and Baylis Medical; and is a consultant for 4C Medical. M. Reardon is a consultant for Medtronic, Boston Scientific, Abbott, and W.L. Gore & Associates. G. Bruschi is a proctor and consultant for Medtronic and Abbott. P.B. Shah is a proctor and consultant for Edwards Lifesciences; is an advisory board member for Xenter; and has received educational grants from Medtronic, Abbott, and Edwards Lifesciences. N. M. Van Mieghem has received research grant support from Abbott, Boston Scientific, Medtronic, Edwards Lifesciences, Daiichi Sankyo, and PulseCath BV. J. Kempfert has served as a proctor for Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. R. Lange is a consultant for Medtronic. D. Atkins has received speaker honoraria from Medtronic. B. K. Whisenant is a consultant for Edwards Lifesciences. M. A. Borger discloses that his hospital receives speaker honoraria and/or consulting fees on his behalf from Edwards Lifesciences, Medtronic, Abbott, and CryoLife. L. Pirelli is a proctor for and has received speaker honoraria from Edwards Lifesciences; and is a consultant for Medtronic. C. Hagl has received speaker honoraria from Edwards Lifesciences. M. W. A. Chu has received speaker honoraria from Medtronic, Edwards Lifesciences, and Terumo Aortic. M. H. Salinger has served as a consultant for Boston Scientific and Edwards Lifesciences. C. Shults is a physician proctor, consultant and has received speaker honoraria for AtriCure and Terumo Aortic. W. Ben Ali has received research grants from Edwards Lifesciences and Medtronic. R. Ibrahim is a proctor for Abbott and Boston Scientific, and a consultant for Abbott, Boston Scientific, Medtronic, and Occlutech. J. Rodés-Cabau has received institutional research grants from and is a consultant for Edwards Lifesciences, Medtronic, and Boston Scientific. F. Maisano has received grant and/or research support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific, New Valve Technology, and Terumo; and has received consulting fees and honoraria from Abbott, Medtronic, Edwards Lifesciences, and SwissVortex. P. M. Ouzounian serves as a consultant for Medtronic and is on their North American advisory board. O. D. Bhadra has received travel compensation from Edwards Lifesciences. R. Estevez-Loureiro has received speaker honoraria and is a proctor for Abbott, Edwards Lifesciences, and Boston Scientific. M. Wyler von Ballmoos has served as a consultant for LivaNova, Medtronic, and Boston Scientific. S. Fukuhara is a consultant for Terumo Aortic. G. M. Deeb is on the Surgical Advisory Board for Medtronic and a member of the Executive, Screening and Steering Committees for the Medtronic FDA TAVR Trials. T. Al-Atassi has served on the advisory board for Edwards Lifesciences, Medtronic, and Abbott; and he has proctored for Edwards Lifesciences. J. E. Bavaria is a consultant for Edwards Lifesciences and Medtronic; and has served as a clinical trial principal investigator for Edwards Lifesciences, Medtronic, and Abbott; he has also served as the Chairman of the Data Safety Monitoring Board for Abbott. A. Geirsson is a member of Medtronic Strategic Surgical Advisory Board. M. Taramasso has been a consultant for Abbott, Boston Scientific, Edwards Lifesciences, 4tech, Mitraltech, Simulands, MTEx, Occlufit, CoreMedic, and Shenqi Medical. M. Gennari is a consultant for Medtronic. The other authors/collaborators have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

