Abstract
Background: Treatment of aortic stenosis in patients with small annuli is challenging and can result in prosthesis-patient mismatch (PPM).
Aims: We aimed to compare the forward flow haemodynamics and clinical outcomes of contemporary transcatheter valves in patients with small annuli.
Methods: The TAVI-SMALL 2 international retrospective registry included 1,378 patients with severe aortic stenosis and small annuli (annular perimeter <72 mm or area <400 mm2) treated with transfemoral self-expanding (SEV; n=1,092) and balloon-expandable valves (BEV; n=286) in 16 high-volume centres between 2011 and 2020. Analyses comparing SEV versus BEV and supra-annular (SAV; n=920) versus intra-annular valves (IAV; n=458) included inverse probability of treatment weighting (IPTW). The primary endpoints were the predischarge mean aortic gradient and incidence of severe PPM. The secondary endpoint was the incidence of more than mild paravalvular leak (PVL).
Results: The predischarge mean aortic gradient was lower after SAV versus IAV (7.8±3.9 vs 12.0±5.1; p<0.001) and SEV versus BEV implantation (8.0±4.1 vs 13.6±4.7; p<0.001). Severe PPM was more common with IAV and BEV when compared to SAV and SEV implantation, respectively, (8.8% vs 3.6%; p=0.007 and 8.7% vs 4.6%; p=0.041). At multivariable logistic regression weighted by IPTW, SAV protected from severe PPM regardless of its definition. More than mild PVL occurred more often with SEV versus BEV (11.6% vs 2.6%; p<0.001).
Conclusions: In small aortic annuli, implantation of SAV and SEV was associated with a more favourable forward haemodynamic profile than after IAV and BEV implantation, respectively. More than mild PVL was more common after SEV than BEV implantation.
Introduction
Prosthesis-patient mismatch (PPM) is present when the effective area of a prosthetic valve inserted into a patient is inferior to that of a normal human valve; the haemodynamic consequence of a valve that is too small compared with the size of the patient’s body is the generation of higher than expected transprosthetic gradients1. The incidence of PPM in patients undergoing transcatheter aortic valve implantation (TAVI) tends to be lower than in patients undergoing surgical aortic valve replacement (SAVR) and is reported to be between 6 and 46% for moderate PPM and between 0 and 15% for severe PPM23. In this setting, self-expanding valves (SEV) were shown to provide a more favourable forward haemodynamic profile compared to balloon-expandable valves (BEV), possibly thanks to the supra-annular leaflet position of most SEV45. A specific focus on patients with small aortic annuli stems from the fact that these patients showed the greatest benefit in terms of haemodynamics when treated with TAVI as compared to SAVR6. Similarly to the overall population, the haemodynamic advantage of TAVI in this subgroup of patients is particularly evident after SEV implantation78. Nonetheless, while evidence of the prognostic relevance of PPM after SAVR is well described, its clinical impact in patients undergoing TAVI remains debatable25910.
In this context, the relative performance of currently available transcatheter heart valves (THV) has not been investigated thoroughly. The aim of this study was to compare the haemodynamics and clinical outcomes of contemporary prostheses in patients with severe aortic stenosis and small annuli treated with TAVI.
Methods
Study design and definition
The observational, retrospective TAVI-SMALL 2 registry included a total of 1,378 patients with severe native aortic valve stenosis and small aortic annuli (defined as an annular area <400 mm2 and/or annular perimeter <72 mm on computed tomography) treated with transfemoral implantation of current-generation SEV (Evolut R and Evolut PRO [Medtronic]; ACURATE neo [Boston Scientific]; Portico [Abbott Vascular]) and BEV (SAPIEN 3 [Edwards Lifesciences]) at 16 high-volume centres (Supplementary Figure 1) between June 2011 and April 2020. This study complied with the Declaration of Helsinki and was approved by local ethics committees. All patients provided written informed consent for the procedure and subsequent data collection, based on local practice and/or local institutional review board approval.
Inclusion criteria were implantation via the transfemoral route of current-generation transcatheter heart valves in native aortic stenosis (both tricuspid and non-tricuspid anatomies) in patients with small aortic annuli. Exclusion criteria were valve-in-valve procedures, TAVI for pure aortic regurgitation and lack of preprocedural computed tomographic data.
Local multidisciplinary Heart Teams evaluated all patients and confirmed the indications for TAVI. All patients underwent preprocedural screening by means of clinical assessment (patient demographic features, New York Heart Association [NYHA] Functional Class, history of angina and/or syncope, comorbidities, laboratory examinations, surgical risk, and frailty evaluation), echocardiography and computed tomography. Aortic annular, leaflet, and left ventricular outflow tract calcifications were classified and graded using a semiquantitative scoring system, as previously described11. Also, computed tomography-derived annular eccentricity (maximum/minimum annular diameter) and percentage of oversizing according to the perimeter ([SEV perimeter/annulus perimeter–1]/100) and area ([BEV area/annulus area–1]/100) were calculated. Prosthesis type and size selection, as well as implantation technique and subsequent antithrombotic therapy, were left to the discretion of the treating physician at each centre.
The rationale of the study was to evaluate the impact of different prosthesis designs on transvalvular haemodynamics and clinical outcomes. Analyses were thus performed according to the mechanism of valve implantation, i.e., SEV (n=1,092: in particular Evolut R/Pro, n=750; ACURATE neo, n=170; and Portico, n=172) versus BEV (SAPIEN 3, n=286), and according to leaflet position, i.e., supra-annular valve (SAV; including Evolut R/Pro and ACURATE neo, n=920) versus intra-annular valve (IAV; including SAPIEN 3 and Portico, n=458). Additional analyses per implanted prosthesis were also performed.
Endpoints
Primary endpoints were the predischarge mean aortic gradient and incidence of severe PPM. PPM was defined as an indexed effective orifice area (EOA) <0.85 cm2/m2 in patients with a body mass index (BMI) <30 kg/m2; those with PPM were further divided into moderate (indexed EOA 0.65-0.85 cm2/m2) or severe PPM (indexed EOA <0.65 cm2/m2) groups. Indexed EOA <0.70 cm2/m2 and <0.55 cm2/m2 were the adjusted thresholds used for moderate and severe PPM, respectively, in patients with a BMI ≥30 kg/m2, as per Valve Academic Research Consortium 3 endpoint definitions12. Additional analyses of PPM without BMI adjustment were also conducted. The EOA was calculated at predischarge transthoracic echocardiography with the continuity equation method; stroke volume was estimated via the left ventricular outflow tract (LVOT) diameter (outer-to-outer border of the valve stent) and velocity-time integral measured just underneath the ventricular margin of the valve stent12. The secondary endpoint was the incidence of predischarge more than mild paravalvular leak (PVL).
Statistical analysis
Continuous variables are reported as mean±standard deviation or median±interquartile range, and were compared using the Student’s t-test or the Mann-Whitney U test (or Wilcoxon rank-sum test) in case of 2-group comparisons on the basis of normality of data distribution and verified using the Shapiro-Wilk test. In case of continuous variable comparisons between more than 2 groups, analysis of variance was performed; Bartlett’s test for equal variances was performed to assess if the variances were comparable between groups, and the Bonferroni correction was applied to adjust for multiple comparisons. Categorical variables are reported as percentage (number) and were compared using the chi-square test, without Yates’ correction for continuity, or Fisher’s exact test, as appropriate. Unadjusted survival curves for all-cause mortality were constructed with the use of Kaplan-Meier estimates and compared with the log-rank test. To account for selection bias between SAV- and IAV-treated patients and between SEV- and BEV-treated patients, a propensity score methodology with inverse probability of treatment weighting (IPTW) was performed1314. Propensity scores predicting each patient’s probability of undergoing TAVI with SAV or IAV and TAVI with SEV or BEV, respectively, were estimated with multivariable logistic regression including variables with a difference in their distribution between the treatment groups or deemed to be clinically relevant. The following covariates were included in the models used to estimate the propensity scores: age, BMI, sex, hypertension, chronic obstructive pulmonary disease (COPD), cerebrovascular disease, coronary artery disease, previous pacemaker (PM) or implantable cardioverter defibrillator (ICD) implant, NYHA Class III or IV, Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM), preprocedural mean aortic valve (AV) gradient, ejection fraction and AV annular perimeter. Stabilised weights were computed from propensity scores by means of IPTW. The weight for SAV treatment was the inverse of the respective propensity score, whereas the weight for IAV treatment was the inverse of 1−propensity score. The weights for SEV and BEV treatment were calculated in the same way, starting from the respective propensity score. Post-IPTW adjustment, the balance of covariates between the treatment groups was assessed by means of standardised mean differences (SMD), and variables were considered balanced if the SMD was ≤10%13. Logistic regression models evaluating the impact of SAV versus IAV and of SEV versus BEV on severe PPM, severe PPM (non-BMI-adjusted) and more than mild PVL were weighted by IPTW, and IPTW-adjusted odds ratios (OR) with 95% confidence intervals (CI) were reported. Cox regression models evaluating the impact of SAV versus IAV and of SEV versus BEV on all-cause mortality were weighted using IPTW. The proportionality assumption was verified using the Schoenfeld residuals method. Adjusted Kaplan-Meier survival curves were generated by weighting the survival function with the IPTW in the 2 comparisons. Doubly robust IPTW adjustment was also performed, augmenting the logistic regression models with covariates that either were unbalanced after the initial IPTW adjustment (SMD >10%) or were considered clinically relevant for the outcome of interest (severe PPM)15. Considering the relatively low number of events, the variables of interest were added separately to the IPTW-adjusted models in order to avoid overfitting.
Clinical follow-up was censored at the date of death or latest available follow-up. Data for patients lost to follow-up were censored at the time of the last contact. A two-sided p-value <0.05 was considered statistically significant. Statistical analyses were performed using Stata version 16.0 (StataCorp).
Results
Study population and clinical features
Baseline characteristics of patients stratified according to both the mechanism of valve expansion and leaflet position are reported in Table 1. Treated patients were mostly female (89%), had a mean age of 83±6 years and were at moderate surgical risk (STS-PROM 5.7±4.0%). Weight and body surface area (BSA) were higher in patients with IAV and BEV versus SAV and SEV, respectively (p<0.001), as was BMI. Small, although statistically significant, differences were noted among groups with regard to clinical variables, such as hypertension, cerebrovascular disease, coronary artery disease, NYHA Functional Class at baseline, previous percutaneous coronary intervention, COPD, angina, atrial fibrillation and previous PM or ICD implantation. Supplementary Table 1 includes baseline characteristics of the cohorts stratified according to the single prosthesis implanted.
Table 1. Baseline demographic characteristics according to leaflet position and mechanism of valve expansion.
| Characteristic | Overall (n=1,378) | Supra-annular valve (n=920) | Intra-annular valve (n=458) | p-value (supra-annular vs intra-annular) | Self-expanding valve (n=1,092) | Balloon-expandable valve (n=286) | p-value (self-expanding vs balloon-expandable) |
|---|---|---|---|---|---|---|---|
| Age, years | 82.9±6.2 | 83.0±6.2 | 82.6±6.2 | 0.239 | 83.0±6.2 | 82.5±6.5 | 0.291 |
| Female | 89.5 (1,233) | 89.3 (822) | 89.7 (411) | 0.824 | 89.6 (979) | 88.8 (254) | 0.680 |
| Weight, kg | 64.7±14.7 | 63.5±14.2 | 67.0±15.5 | <0.001 | 63.9±14.2 | 67.5±16.4 | <0.001 |
| Height, cm | 157.7±7.7 | 157.3±7.7 | 158.5±7.7 | 0.006 | 157.4±7.4 | 159.1±8.5 | 0.001 |
| Body surface area, m² | 1.65±0.19 | 1.63±0.19 | 1.68±0.19 | <0.001 | 1.64±0.18 | 1.69±0.21 | <0.001 |
| Body mass index, kg/m² | 25.9±5.4 | 25.6±5.3 | 26.6±5.6 | <0.001 | 25.7±5.3 | 26.6±5.8 | 0.018 |
| Hypertension | 85.5 (1,177) | 84.1 (773) | 88.2 (404) | 0.042 | 84.5 (922) | 89.2 (255) | 0.047 |
| Diabetes mellitus | 26.4 (364) | 25.4 (234) | 28.4 (130) | 0.242 | 26.3 (287) | 26.9 (77) | 0.827 |
| Dyslipidaemia | 51.8 (712) | 53.2 (488) | 49.1 (224) | 0.159 | 52.7 (574) | 48.4 (138) | 0.197 |
| COPD | 11.5 (158) | 11.0 (101) | 12.5 (57) | 0.417 | 10.3 (112) | 16.1 (46) | 0.006 |
| Peripheral artery disease or previous PTA | 11.7 (156) | 12.6 (113) | 9.7 (43) | 0.114 | 12.2 (130) | 9.5 (26) | 0.214 |
| Cerebrovascular disease | 10.4 (143) | 8.7 (80) | 13.8 (63) | 0.004 | 9.3 (101) | 14.7 (42) | 0.007 |
| Previous PCI | 21.9 (301) | 20.3 (186) | 25.3 (115) | 0.035 | 21.4 (233) | 23.9 (68) | 0.355 |
| Previous CABG | 6.0 (82) | 5.6 (51) | 6.8 (31) | 0.365 | 5.6 (61) | 7.3 (21) | 0.268 |
| Previous MI | 9.5 (128) | 9.1 (81) | 10.3 (47) | 0.464 | 9.3 (99) | 10.2 (29) | 0.656 |
| Coronary artery disease | 38.2 (525) | 35.9 (329) | 42.9 (196) | 0.012 | 36.0 (391) | 46.8 (134) | 0.001 |
| PM or ICD | 11.4 (157) | 10.4 (96) | 13.3 (61) | 0.112 | 10.6 (116) | 14.3 (41) | 0.079 |
| Atrial fibrillation | 29.4 (269) | 31.3 (182) | 26.2 (87) | 0.106 | 31.6 (225) | 21.9 (44) | 0.008 |
| Angina | 20.2 (230) | 19.2 (165) | 23.4 (65) | 0.134 | 19.0 (185) | 27.8 (45) | 0.010 |
| NYHA Class III or IV | 67.4 (929) | 65.6 (604) | 71.0 (325) | 0.048 | 66.5 (726) | 71.0 (203) | 0.149 |
| STS-PROM, % | 5.7±4.0 | 5.9±4.3 | 5.5±3.3 | 0.097 | 5.7±4.1 | 5.7±3.6 | 0.951 |
| Values are mean±standard deviation or % (n). The values in bold represent differences between groups with p<0.100. CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; ICD: implantable cardioverter-defibrillator; MI: myocardial infarction; NYHA: New York Heart Association; PTA: percutaneous transluminal angioplasty; PCI: percutaneous coronary intervention; PM: pacemaker; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality | |||||||
Echocardiographic and computed tomography features
Baseline echocardiographic and computed tomography features are shown in Table 2. Slightly higher preprocedural mean and peak aortic gradients and lower measured EOA were present in SAV versus IAV and SEV versus BEV cohorts, respectively, (p<0.001). The SAV and SEV groups also had lower ejection fractions (58±11% vs 61±10% and 58±11% vs 62±10%; both p<0.001) and a higher prevalence of baseline moderate or more aortic regurgitation, mitral regurgitation or tricuspid regurgitation when compared with IAV and BEV, respectively, while bicuspid valves were less common in the two former cohorts (3.4% vs 5.8%; p=0.053 and 3.5% vs 7.3%; p=0.007). Computed tomography-derived mean diameters and the area- and perimeter-derived diameters slightly differed between groups, with a trend to wider eccentricity in the IAV and BEV groups. Severe annular and LVOT calcifications were more frequent among patients with SAV and SEV, while severe leaflet calcifications differed only when comparing SEV and BEV. On the other hand, porcelain aorta was more common in IAV versus SAV and BEV versus SEV, respectively. Baseline echocardiographic and computed tomography features of single prosthesis cohorts are reported in Supplementary Table 2.
Table 2. Baseline echocardiographic and computed tomography characteristics according to leaflet position and mechanism of valve expansion.
| Characteristic | Overall (n=1,378) | Supra-annular valve (n=920) | Intra-annular valve (n=458) | p-value (supra-annular vs intra-annular) | Self-expanding valve (n=1,092) | Balloon-expandable valve (n=286) | p-value (self-expanding vs balloon-expandable) |
|---|---|---|---|---|---|---|---|
| Mean AV gradient, mmHg | 47.7±16.0 | 49.0±16.1 | 45.3±15.5 | <0.001 | 48.6±16.1 | 44.3±15.3 | <0.001 |
| Maximum AV gradient, mmHg | 77.6±24.8 | 80.0±24.7 | 72.9±24.2 | <0.001 | 79.0±24.7 | 72.3±24.4 | <0.001 |
| EOA, cm2 | 0.64±0.21 | 0.63±0.18 | 0.66±0.25 | 0.023 | 0.64±0.19 | 0.67±0.27 | 0.034 |
| sPAP, mmHg | 40.3±13.7 | 39.6±13.0 | 41.9±15.1 | 0.012 | 40.1±13.3 | 41.5±15.2 | 0.189 |
| TAPSE, mm | 20.9±3.6 | 21.0±3.7 | 20.3±2.9 | 0.076 | 21.0±3.6 | 20.0±2.9 | 0.059 |
| Bicuspid AV | 4.3 (49) | 3.4 (24) | 5.8 (25) | 0.053 | 3.5 (30) | 7.3 (19) | 0.007 |
| Moderate or greater AR | 6.7 (83) | 8.3 (67) | 3.8 (16) | 0.003 | 7.6 (74) | 3.4 (9) | 0.017 |
| Moderate or greater MR | 8.8 (112) | 10.4 (88) | 5.6 (24) | 0.004 | 10.4 (105) | 2.6 (7) | <0.001 |
| Moderate or greater TR | 6.9 (74) | 8.1 (54) | 5.0 (20) | 0.056 | 7.8 (65) | 3.8 (9) | 0.033 |
| Ejection fraction, % | 59.2±10.7 | 58.1±10.9 | 61.4±9.9 | <0.001 | 58.4±10.6 | 62.2±10.2 | <0.001 |
| LVEF <40% | 5.1 (71) | 6.1 (56) | 3.3 (15) | 0.026 | 5.7 (62) | 3.1 (9) | 0.085 |
| CT data | |||||||
| Mean annular diameter, mm | 21.2±1.3 | 21.2±1.4 | 21.3±1.1 | 0.084 | 21.2±1.3 | 21.4±1.0 | 0.005 |
| Maximum diameter, mm | 23.7±1.8 | 23.6±1.9 | 23.9±1.6 | <0.001 | 23.6±1.9 | 24.0±1.4 | 0.005 |
| Minimum diameter, mm | 18.8±1.8 | 18.8±1.9 | 18.7±1.6 | 0.457 | 18.7±1.9 | 18.9±1.5 | 0.212 |
| Annular eccentricity | 1.27±0.17 | 1.27±0.17 | 1.29±0.17 | 0.029 | 1.27±0.17 | 1.28±0.18 | 0.553 |
| Mean aortic annular perimeter, mm | 66.9±4.3 | 67.3±3.6 | 66.0±5.3 | <0.001 | 67.4±3.6 | 65.0±5.9 | <0.001 |
| Mean aortic annular area, mm2 | 350.1±34.4 | 347.1±35.5 | 355.5±31.6 | <0.001 | 346.5±35.1 | 362.3±28.7 | <0.001 |
| Area-derived diameter, mm | 21.1±1.1 | 21.0±1.1 | 21.3±1.0 | <0.001 | 21.0±1.1 | 21.5±0.9 | <0.001 |
| Perimeter-derived diameter, mm | 21.3±1.5 | 21.4±1.2 | 21.0±1.7 | <0.001 | 21.5±1.1 | 20.7±1.9 | <0.001 |
| Severe leaflet calcification | 19.3 (185) | 18.5 (107) | 20.6 (78) | 0.421 | 17.1 (115) | 24.5 (70) | 0.008 |
| Severe annular calcification | 4.4 (38) | 6.2 (29) | 2.2 (9) | 0.005 | 5.6 (34) | 1.5 (4) | 0.008 |
| Severe LVOT calcification | 4.1 (40) | 5.7 (34) | 1.6 (6) | 0.001 | 5.2 (36) | 1.4 (4) | 0.006 |
| LMCA diameter, mm | 12.5±2.6 | 12.4±2.5 | 12.6±2.7 | 0.184 | 12.3±2.5 | 12.8±2.6 | 0.003 |
| RCA diameter, mm | 14.4±2.8 | 14.2±3.0 | 14.6±2.5 | 0.034 | 14.3±2.9 | 14.7±2.5 | 0.042 |
| Sinotubular junction diameter, mm | 25.9±2.7 | 25.8±2.8 | 26.0±2.5 | 0.144 | 25.9±2.8 | 25.9±2.4 | 0.927 |
| Sinus of Valsalva diameter, mm | 28.7±2.5 | 28.8±2.5 | 28.6±2.5 | 0.180 | 28.9±2.5 | 28.4±2.5 | 0.013 |
| Ascending aorta diameter, mm | 31.9±3.9 | 31.6±3.9 | 32.3±3.9 | 0.016 | 31.9±4.0 | 32.0±3.8 | 0.657 |
| Porcelain aorta | 5.1 (61) | 2.7 (21) | 9.4 (40) | <0.001 | 3.2 (30) | 11.9 (31) | <0.001 |
| Values are mean±standard deviation or % (n). The values in bold represent differences between groups with p<0.100. AR: aortic regurgitation; AV: aortic valve; CT: computed tomography; EOA: effective orifice area; LMCA: left main coronary artery; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; MR: mitral regurgitation; RCA: right coronary artery; RV: right ventricular; sPAP: systolic pulmonary artery pressure; TAPSE: tricuspid annular plane systolic excursion; TR: tricuspid regurgitation | |||||||
Procedural features
Procedural data are shown in Table 3. With respect to prosthesis selection, a higher proportion of THV with a nominal diameter of 25 mm or less were implanted among the IAV and BEV groups (30.8% vs 95.2% [SAV vs IAV] and 39.9% vs 98.9% [SEV vs BEV]; p<0.001). When compared with IAV and BEV, SAV and SEV, respectively, had higher proportions of oversizing ≥15% (64.2% vs 34.3% and 61.0% vs 28.7%; p<0.001). The proportion of predilation was higher in IAV versus SAV (46.9% vs 39.5%; p<0.001) and SEV versus BEV (44.3% vs 32.9%; p<0.001). On the other hand, post-dilation was more common in the SAV (31.9% vs 19.6% in IAV; p<0.001) and SEV (32.9% vs 8.2% in BEV; p<0.001) groups. As shown in Supplementary Table 3, the ACURATE neo and Portico cohorts presented the highest rates of predilation (65.7% and 70.0%) and post-dilation (36.5% and 38.2%). No difference in the incidence of annular rupture was observed.
Table 3. Procedural characteristics according to leaflet position and mechanism of valve expansion.
| Characteristic | Overall (n=1,378) | Supra-annular valve (n=920) | Intra-annular valve (n=458) | p-value (supra-annular vs intra-annular) | Self-expanding valve (n=1,092) | Balloon-expandable valve (n=286) | p-value (self-expanding vs balloon-expandable) |
|---|---|---|---|---|---|---|---|
| Valve size 25 mm or less | 52.2 (719) | 30.8 (283) | 95.2 (436) | <0.001 | 39.9 (436) | 98.9 (283) | <0.001 |
| Oversizing by perimeter | 15.0±8.7 | 17.5±7.2 | 11.2±9.9 | <0.001 | 17.0±7.1 | 9.5±11.4 | <0.001 |
| Oversizing by perimeter ≥15% | 54.1 (745) | 64.2 (591) | 33.6 (154) | <0.001 | 61.0 (666) | 27.6 (69) | <0.001 |
| Oversizing by area | 36.9±21.2 | 45.5±18.4 | 22.0±17.3 | <0.001 | 44.4±17.7 | 11.9±10.8 | <0.001 |
| Oversizing by area ≥15% | 82.6 (1,138) | 96.3 (886) | 55.0 (252) | <0.001 | 96.7 (1,056) | 28.7 (82) | <0.001 |
| Oversizing ≥15% | 54.3 (748) | 64.2 (591) | 34.3 (157) | <0.001 | 61.0 (666) | 28.7 (82) | <0.001 |
| Predilation | 41.9 (573) | 39.5 (361) | 46.9 (212) | 0.009 | 44.3 (481) | 32.9 (92) | 0.001 |
| Post-dilation | 27.8 (380) | 31.9 (292) | 19.6 (88) | <0.001 | 32.9 (357) | 8.2 (23) | <0.001 |
| Annular rupture | 0.3 (4) | 0.2 (2) | 0.4 (2) | 0.548 | 0.3 (3) | 0.3 (1) | 0.909 |
| Values are mean±standard deviation or % (n). The values in bold represent differences between groups with p<0.100. Oversizing ≥15% refers to oversizing by perimeter ≥15% for self-expanding valves and oversizing by area ≥15% for balloon-expandable valves. | |||||||
Procedural and clinical outcomes
Clinical and procedural outcomes are reported in Table 4. The mean aortic valve gradients were higher in the IAV and BEV cohorts (7.8 vs 12.0 mmHg [SAV vs IAV] and 8.0 vs 13.6 mmHg [SEV vs BEV]; p<0.001). This was accompanied by a higher incidence of severe PPM with IAV and BEV (3.6% vs 8.8%; p=0.007 and 4.6% vs 8.7%; p=0.041) (Central illustration), in turn paralleled by a higher proportion of severe PPM with no BMI adjustment, moderate PPM and any degree of PPM (p<0.001). The SMDs before and after covariate balancing with the IPTW method are illustrated in Figure 1 and Supplementary Table 4. After IPTW adjustment, SAV implantation remained a stronger protective factor for the development of severe PPM than SEV implantation (p=0.002 and p=0.029, respectively). On the other hand, SAV alone protected from severe PPM (non-BMI-adjusted) (Table 5), and doubly robust analyses were more consistent with SAV than with SEV (Supplementary Table 5). Among single THV patients, those with Portico and SAPIEN 3 had the highest mean aortic valve gradients (9.2±4.5 and 13.6±4.7 mmHg; overall p<0.001) and incidence of severe PPM (9.0% and 8.7%; overall p=0.058) (Supplementary Table 6, Supplementary Figure 2).
Acute complications were rare, with no differences between groups with regard to vascular complications or major bleeding events. More than mild PVL was more common after SEV versus BEV implantation (11.6% vs 2.6%; p<0.001) (Central illustration), while more than moderate PVL was more common after SAV versus IAV (p=0.043) and SEV versus BEV (p=0.052). SEV, but not SAV, implantation increased the risk of more than mild PVL after IPTW adjustment (Table 5). Of note, the highest incidence of more than mild PVL was observed with Portico (19.0% vs 9.9% Evolut R/Pro, 11.2% ACURATE neo and 2.6% SAPIEN 3; p<0.001). When compared with BEV, SEV recipients had a higher incidence of permanent pacemaker implantation (PPI) and second valve implantation (13.5% vs 8.1%; p=0.013 and 2.0% vs 0.3%; p=0.065, respectively). When comparing single prostheses, patients with the SAPIEN 3 had the lowest incidence of PPI (8.1%; p=0.039) (Supplementary Table 6).
At a median follow-up of 377 days (interquartile range 168-700 days), no differences were observed between patients in the SAV versus IAV and SEV versus BEV cohorts in terms of all-cause mortality (9.4% vs 11.9%; p=0.172 and 9.8% vs 12.3%; p=0.218). When compared with SAV at Kaplan-Meier analysis, the use of IAV did not result in an increased risk of all-cause mortality (p=0.748). Similarly, no difference in all-cause mortality was observed between SEV and BEV (p=0.687) at the time-to-event analysis. Results were confirmed when comparing single-prosthesis cohorts (p=0.667) (Supplementary Figure 3). No significant differences in all-cause mortality were present when comparing SAV versus IAV and SEV versus BEV at Cox regression analysis, neither before nor after IPTW adjustment (Table 5, Figure 2). A trend towards decreased cardiovascular mortality was observed when comparing SAV and IAV (2.8% vs 4.5%; p=0.099), with the only significant difference at analysis per single prosthesis present when comparing Evolut R/Pro with Portico (2.7% vs 5.4%; p=0.021) (Supplementary Table 6).
The incidence of myocardial infarction, stroke or transient ischaemic attack and hospitalisation for heart failure did not differ between groups. Acute kidney injury was more common after ACURATE neo and Portico implantations (p=0.020) (Supplementary Table 6) in the analysis per single prosthesis.
Table 4. Post-procedural characteristics and follow-up according to leaflet position and mechanism of valve expansion.
| Characteristic | Overall (n=1,378) | Supra-annular valve (n=920) | Intra-annular valve (n=458) | p-value (supra-annular vs intra-annular) | Self-expanding valve (n=1,092) | Balloon-expandable valve (n=286) | p-value (self-expanding vs balloon-expandable) |
|---|---|---|---|---|---|---|---|
| Predischarge | |||||||
| Any vascular complication | 14.0 (192) | 13.6 (124) | 14.8 (68) | 0.545 | 14.2 (154) | 13.3 (38) | 0.678 |
| Major vascular complication | 4.7 (65) | 4.4 (40) | 5.5 (25) | 0.386 | 4.5 (49) | 5.6 (16) | 0.453 |
| Need for second valve implantation | 1.7 (23) | 1.7 (16) | 1.5 (7) | 0.770 | 2.0 (22) | 0.3 (1) | 0.065 |
| Mean AV gradient, mmHg | 9.3±4.8 | 7.8±3.9 | 12.0±5.1 | <0.001 | 8.0±4.1 | 13.6±4.7 | <0.001 |
| Maximum AV gradient, mmHg | 16.5±8.2 | 14.5±6.8 | 22.4±8.9 | <0.001 | 14.8±7.1 | 24.8±7.7 | <0.001 |
| EOA, cm2 | 1.61±0.45 | 1.74±0.50 | 1.47±0.34 | <0.001 | 1.72±0.49 | 1.41±0.29 | <0.001 |
| Indexed EOA, cm2/m2 | 1.00±0.30 | 1.11±0.31 | 0.88±0.23 | <0.001 | 1.08±0.31 | 0.84±0.19 | <0.001 |
| Any PPM (non-BMI-adjusted) | 33.6 (211) | 16.9 (56) | 52.4 (155) | <0.001 | 20.5 (84) | 58.3 (127) | <0.001 |
| Any PPM | 28.0 (176) | 13.5 (45) | 44.3 (131) | <0.001 | 16.6 (68) | 49.5 (108) | <0.001 |
| Moderate PPM (non-BMI-adjusted) | 25.0 (157) | 12.6 (42) | 38.8 (115) | <0.001 | 14.9 (61) | 44.0 (96) | <0.001 |
| Moderate PPM | 22.0 (138) | 9.9 (33) | 35.5 (105) | <0.001 | 11.9 (49) | 40.8 (89) | <0.001 |
| Severe PPM (non-BMI-adjusted) | 8.6 (54) | 4.2 (14) | 13.5 (40) | <0.001 | 5.6 (23) | 14.2 (31) | <0.001 |
| Severe PPM | 6.0 (38) | 3.6 (12) | 8.8 (26) | 0.007 | 4.6 (19) | 8.7 (19) | 0.041 |
| More than mild PVL | 9.4 (107) | 10.1 (73) | 8.3 (34) | 0.315 | 11.6 (100) | 2.6 (7) | <0.001 |
| More than moderate PVL | 1.1 (12) | 1.5 (11) | 0.2 (1) | 0.043 | 1.4 (12) | 0 | 0.052 |
| PPI | 12.4 (169) | 13.2 (120) | 10.7 (49) | 0.187 | 13.5 (146) | 8.1 (23) | 0.013 |
| BARC major bleeding | 5.9 (81) | 6.4 (59) | 4.8 (22) | 0.231 | 5.9 (64) | 5.9 (17) | 0.958 |
| Follow-up | |||||||
| All-cause mortality | 10.3 (129) | 9.4 (76) | 11.9 (53) | 0.172 | 9.8 (95) | 12.3 (34) | 0.218 |
| Cardiovascular mortality | 3.4 (42) | 2.8 (22) | 4.5 (20) | 0.099 | 3.2 (31) | 4.0 (11) | 0.537 |
| Myocardial infarction | 1.1 (12) | 1.0 (7) | 1.3 (5) | 0.763 | 1.2 (10) | 0.7 (2) | 0.741 |
| TIA/stroke | 3.3 (36) | 3.9 (28) | 2.3 (8) | 0.182 | 3.6 (29) | 2.6 (7) | 0.404 |
| Acute kidney injury | 2.9 (27) | 3.2 (19) | 2.4 (8) | 0.497 | 3.4 (22) | 1.9 (5) | 0.284 |
| Hospitalisation for HF | 6.2 (65) | 5.9 (42) | 6.8 (23) | 0.598 | 6.1 (48) | 6.6 (17) | 0.770 |
| Values are mean±standard deviation or % (n). The values in bold represent differences between groups with p<0.100. AV: aortic valve; BARC: Bleeding Academic Research Consortium; BMI: body mass index; EOA: effective orifice area; HF: heart failure; PPI: permanent pacemaker implantation; PPM: prosthesis-patient mismatch; PVL: paravalvular leak; TIA: transient ischaemic attack | |||||||
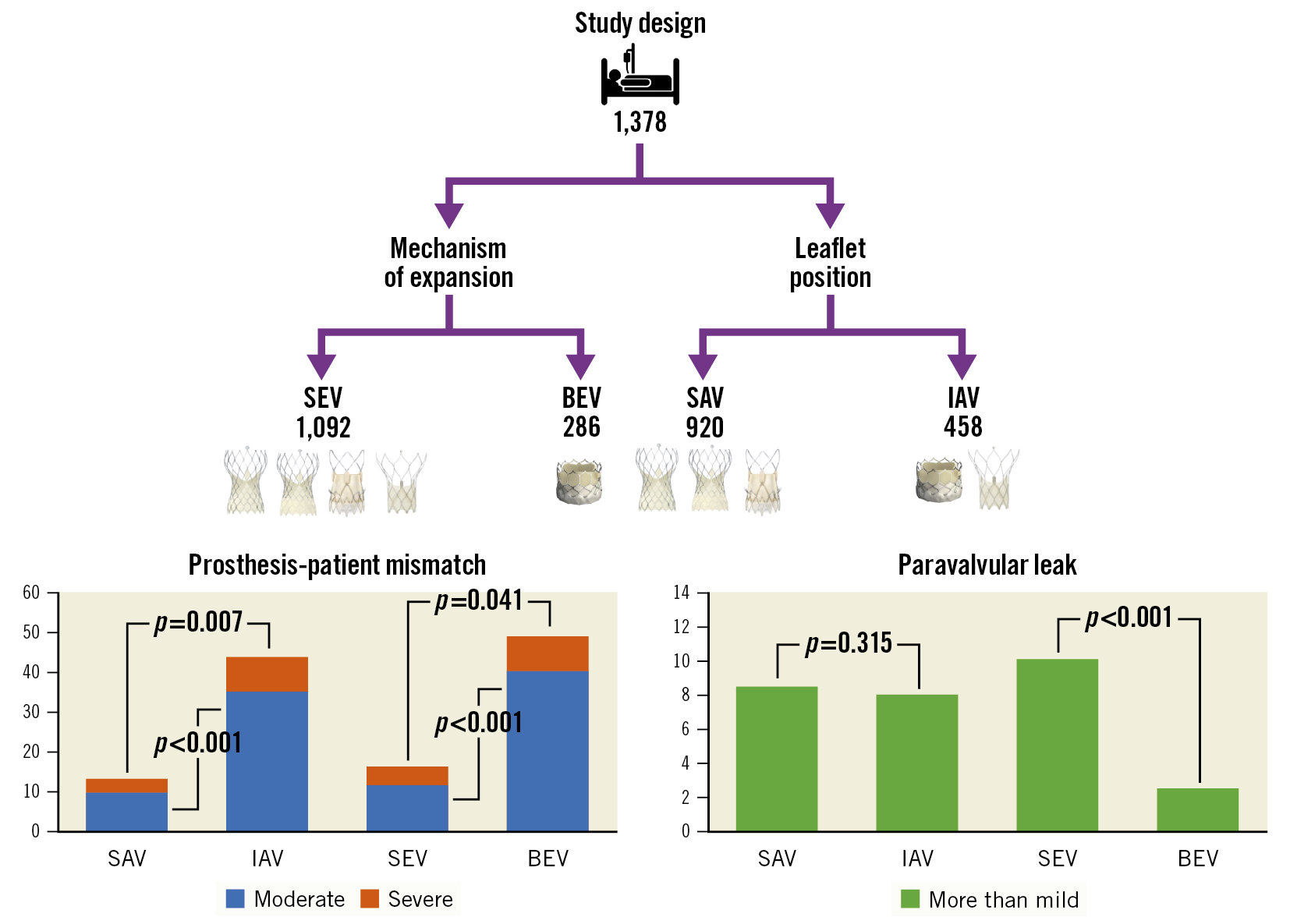
Central illustration. Incidence of PPM and more than mild PVL according to leaflet position and mechanism of valve expansion. BEV: balloon-expandable valve; IAV: intra-annular valve; PPM: prosthesis-patient mismatch; PVL: paravalvular leak; SAV: supra-annular valve; SEV: self-expandable valve
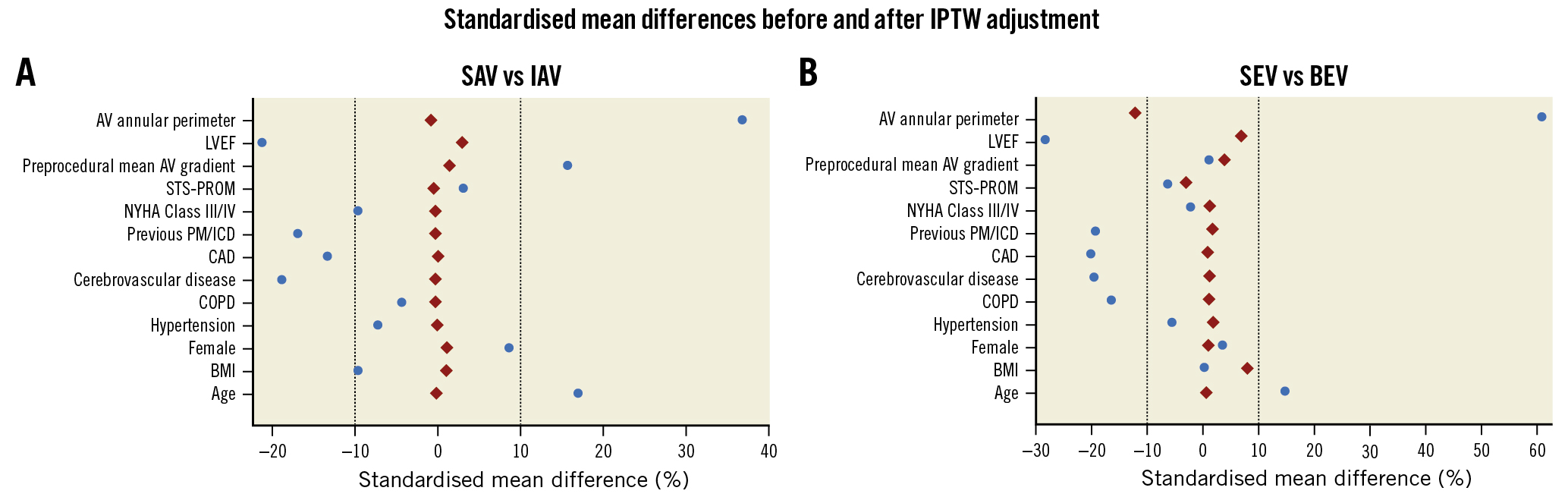
Figure 1. Standardised mean differences (SMDs) of the covariates used for propensity score modelling before and after inverse probability of treatment weighting (IPTW) adjustment for comparisons of SAV versus IAV (A) and SEV versus BEV (B). After adjustment, all covariates showed SMDs within the 10% cut-off (dashed vertical lines), except AV annular perimeter in the SEV versus BEV comparison (−11.2%).AV: aortic valve; BEV: balloon-expandable valve; BMI: body mass index; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; IAV: intra-annular valve; ICD: implantable cardioverter-defibrillator; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; PM: pacemaker; SAV: supra-annular valve; SEV: self-expanding valve; STS-PROM: Society of Thoracic Surgery Predicted Risk of Mortality
Table 5. Unadjusted and adjusted risk of clinical outcomes.
| Characteristic | Overall (n=1,378) | Supra-annular valve (n=920) | Intra-annular valve (n=458) | Unadjusted OR/HR (95% CI)* | p-value | IPTW-adjusted HR/OR (95% CI)† | p-value |
|---|---|---|---|---|---|---|---|
| Severe PPM | 6.0 (38) | 3.6 (12) | 8.8 (26) | 0.22 (0.11-0.44) | <0.001 | 0.25 (0.10-0.60) | 0.002 |
| Severe PPM (non-BMI-adjusted) | 8.6 (54) | 4.2 (14) | 13.5 (40) | 0.28 (0.15-0.53) | <0.001 | 0.36 (0.16-0.82) | 0.015 |
| More than mild PVL | 9.4 (107) | 10.1 (73) | 8.3 (34) | 1.24 (0.81-1.90) | 0.319 | 0.98 (0.60-1.60) | 0.944 |
| All-cause mortality | 10.3 (129) | 9.4 (76) | 11.9 (53) | 1.10 (0.77-1.56)^ | 0.604 | 1.34 (0.81-2.23)^ | 0.255 |
| Characteristic | Overall (n=1,378) | Self-expanding valve (n=1,092) | Balloon-expandable valve (n=286) | Unadjusted OR/HR (95% CI)* | p-value | IPTW-adjusted HR/OR (95% CI)† | p-value |
| Severe PPM | 6.0 (38) | 4.6 (19) | 8.6 (19) | 0.25 (0.13-0.48) | <0.001 | 0.40 (0.18-0.91) | 0.029 |
| Severe PPM (non-BMI-adjusted) | 8.6 (54) | 5.6 (23) | 14.2 (31) | 0.36 (0.20-0.63) | <0.001 | 0.66 (0.33-1.33) | 0.246 |
| More than mild PVL | 9.4 (107) | 11.6 (100) | 2.6 (7) | 4.87 (2.24-10.6) | <0.001 | 4.85 (1.70-13.9) | 0.003 |
| All-cause mortality | 10.3 (129) | 9.8 (95) | 12.3 (34) | 1.26 (0.85-1.87)^ | 0.258 | 1.59 (0.90-2.81)^ | 0.109 |
| Results reported as % (number of events), HR, OR, and 95% CI. Comparisons are SAV versus IAV and SEV versus BEV. *Generated with univariable logistic/Cox regression analysis. †Generated with logistic/Cox regression modelling after IPTW adjustment. ^HR was analysed via Cox regression analysis for the outcome all-cause mortality (at a median follow-up of 377 days). All other outcomes had OR assessed via logistic regression analysis. The values in bold represent differences between groups with p<0.100. AV: aortic valve; BEV: balloon-expandable valve; BMI: body mass index; CI: confidence interval; HR: hazard ratio; IAV: intra-annular valve; IPTW: inverse probability of treatment weighting; OR: odds ratio; PPM: prosthesis-patient mismatch; PVL: paravalvular leak; SAV: supra-annular valve; SEV: self-expanding valve | |||||||
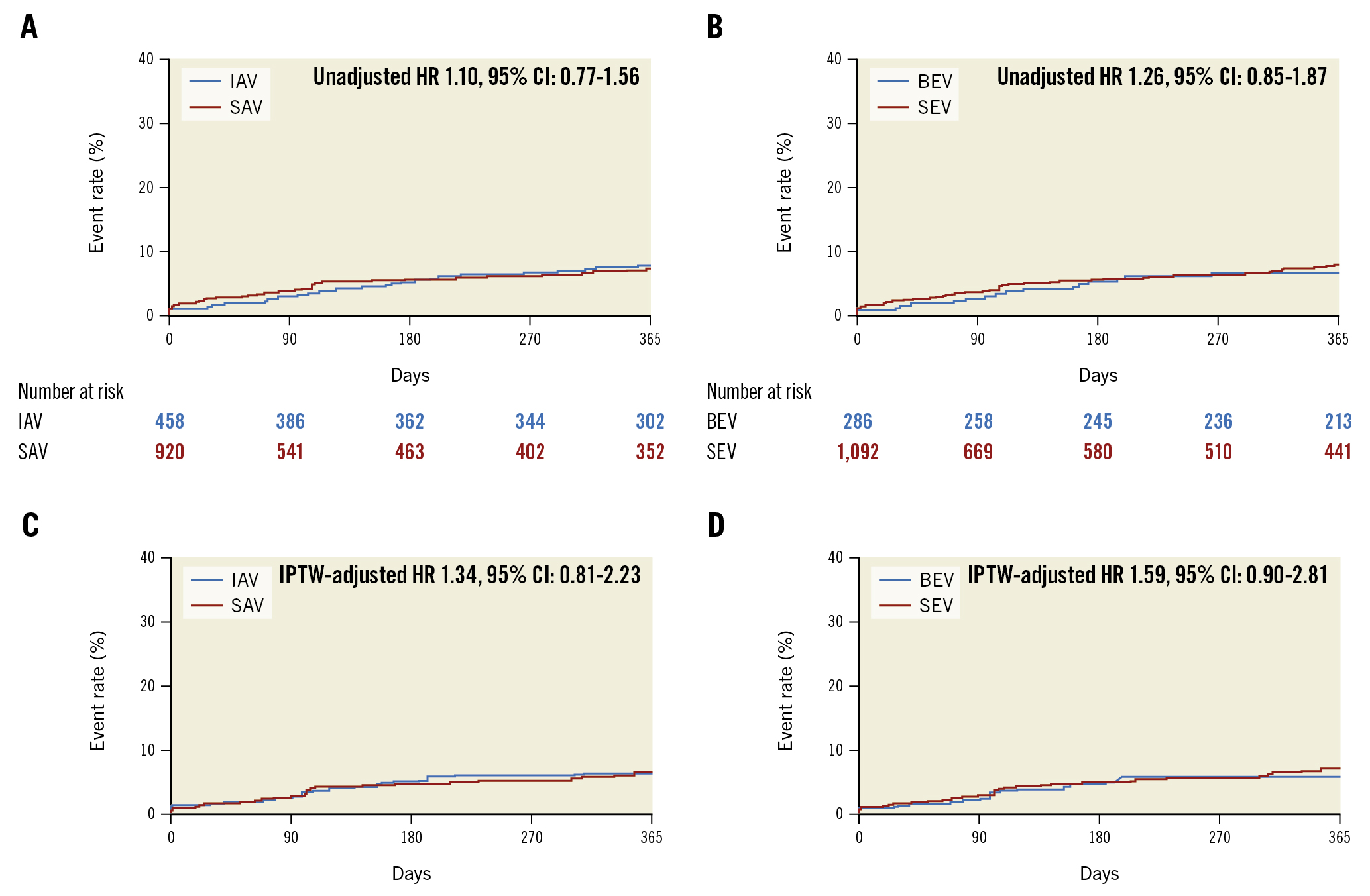
Figure 2. Non-adjusted and IPTW-adjusted Kaplan-Meier curves of all-cause mortality in patients treated with SAV versus IAV and SEV versus BEV. At a median follow-up of 377 (interquartile range 168-700) days, no significant difference in the risk of all-cause mortality was evident in either the non-adjusted comparison of SAV versus IAV (A) and SEV versus BEV (B) the adjusted comparison of SAV versus IAV (C) and SEV versus BEV (D). The number of patients at risk during follow-up is not applicable in the adjusted inverse probability of treatment weight analysis. BEV: balloon-expandable valve; CI: confidence interval; HR: hazard ratio; IAV: intra-annular valve; IPTW: inverse probability of treatment weighting. SAV: supra-annular valve; SEV: self-expanding valve
Discussion
The objective of the present study was to compare the forward flow haemodynamics and clinical outcomes of the currently available THV in patients with severe aortic stenosis and small annuli. The main findings are the following:
⢠IAV and BEV are associated with increased mean aortic valve (AV) gradients and the incidence of severe PPM when compared to SAV and SEV, respectively;
⢠The incidence of more than mild PVL was higher after SEV versus BEV, but not SAV versus IAV implantation;
⢠IPTW-adjusted logistic regression analyses confirmed SAV as a protective factor from severe PPM, regardless of its definition, and BEV as protective factor from more than mild PVL.
Of patients treated with SAVR, up to one-half and one-quarter have PPM and severe PPM, respectively2. A recent meta-analysis conducted on 745 patients described a relative risk reduction of 77% in the incidence of PPM in patients treated with TAVI as compared to surgery9. Nonetheless, not all THV are born equal. Indeed, not only did comparison of SAVR and TAVI with a SAPIEN 3 intra-annular BEV in the PARTNER 3 Trial show similar transvalvular gradients and incidence of severe PPM (4.6 vs 6.3%, respectively; p=0.30)16, but also SAPIEN 3 implantation was identified as an independent predictor of PPM in the OCEAN-TAVI registry17. Notwithstanding the slight, although significant, difference in BSA between groups, the lower mean AV gradients and incidence of severe PPM with SAV versus IAV implantation further clarify the role of leaflet position in the development of PPM in patients with small annuli, in line with previous evidence from the TAVI-SMALL registry, which showed an increased risk of PPM in SEV with intra-annular leaflets810. Similarly, in a recent subanalysis of propensity score-matched patients from the OCEAN-TAVI registry treated with a third-generation THV, Evolut R outperformed SAPIEN 3 in terms of the mean AV gradient18. Schofer et al reported data from 1,309 patients undergoing TAVI with different THV: the lowest rate of severe PPM was present with supra-annular SEV (4%), whereas the highest rate was detected in patients with self-expanding cusp-fixated prostheses (25%) and intra-annular BEV (24%)19. The importance of leaflet position in THV implanted in patients with small annuli has been recently reported in a retrospective registry of 1,069 patients, where a higher incidence of PPM was found after the implantation of intra-annular BEV or intra-annular mechanically expandable THV compared to intra- and supra-annular SEV; SEV implantation itself was linked to a lower incidence of PPM20. The haemodynamic advantage of TAVI with supra-annular valves in patients with small annuli has been addressed in other studies. Indeed, in the CHOICE-Extend registry, the supra-annular SEV (Evolut R) also had higher indexed EOA and lower post-procedural mean gradients and PPM than the intra-annular BEV (SAPIEN 3)7. Another supra-annular SEV, the ACURATE neo, was comparable to the Evolut R, in terms of the incidence of severe PPM, in a recent randomised trial21, which similarly resulted in lower gradients and lower rate of severe PPM when compared with the SAPIEN 3 among 246 propensity score-matched patients with small aortic annuli22. These findings were also confirmed in Japanese patients with very small annuli23. In addition, both oversizing and post-dilation, previously shown to protect from the incidence of PPM10, were more common in SAV and SEV versus IAV and BEV, respectively. Of note, the method used for measuring PPM appears to be important. Indeed, not only did reclassification of PPM using a predicted EOA reveal a lower incidence with respect to a measured EOA-based method in a large cohort treated mainly with BEV, but also a stronger association with high residual gradient was appreciated with predicted versus measured PPM. Further studies will need to be undertaken to adequately address the definition of PPM24.
The lower incidence of more than mild PVL after BEV implantation parallels available randomised evidence2526 and supports the relevance of an external skirt or seal at the inflow portion of the THV, also in patients with small annuli. We expect new prosthesis iterations, namely Navitor (Abbott) (Sondergaard L. 30-day outcomes from a next generation TAVI device with an active sealing cuff. EuroPCR 2021. Paris, France) and the ACURATE neo2 (Boston Scientific),27 to mitigate the rates of PVL. Of note, the observed increased risk of acute kidney injury with the Portico and ACURATE neo might also be related to the increased use of contrast agent and the performance of predilation and post-dilation, undertaken in order to mitigate the rates of PVL28.
The increased risk of PPI after SEV versus BEV is similar to results from direct randomised comparisons in the SOLVE-TAVI, CHOICE and PORTICO-IDE trials2526. Also, the significant difference in the incidence of PPI after implantation of SAPIEN 3 versus Evolut R/Pro or Portico, but not ACURATE neo, confirms the favourable profile of the latter prosthesis among SEV in terms of impact on persistent conduction disturbances after TAVI2129.
The absence of significant differences in all-cause mortality between groups, confirmed at IPTW-adjusted analyses, needs to be acknowledged in light of the non-uniform distribution of patients among groups and the related lack of power in assessing this outcome. These results parallel those from the TAVI-SMALL registry8 and those recently reported in a multicentre analysis of patients with small annuli, where 30-day and 12-month mortality rates were similar between patients treated with the SAPIEN 3, Evolut, ACURATE neo, Portico and Lotus THV20. Of note, numerical differences favouring SAV versus IAV and SEV versus BEV were present, as were differences in cardiovascular mortality when comparing SAV and IAV (2.8% vs 4.5%; p=0.099) and Evolut R/Pro with Portico (2.7% vs 5.4%; p=0.021), although the observational, retrospective nature of the current study represents an additional relevant limitation. Previous 30-day results from head-to-head randomised comparisons of SAV and IAV revealed either no difference in the valve-related efficacy endpoint between groups25 or a higher incidence of the safety and efficacy endpoint in SAV versus IAV29. The possibility that the favourable forward haemodynamic profile linked to SAV implantation might be of prognostic significance in patients with small annuli will need to be further addressed at long-term follow-up analysis and in randomised studies. In this setting, results from the ongoing Small Annuli Randomized to Evolut or SAPIEN Trial (SMART) will be of paramount importance (ClinicalTrials.gov: NCT04722250). No differences between groups were observed at 12 months, in terms of transient ischaemic attack or stroke, myocardial infarction or hospitalisation for heart failure.
Limitations
First, selection and confounding bias cannot be excluded because of the observational nature of our study. Second, underreporting or missing echocardiographic and follow-up data need to be acknowledged. Third, the absence of core laboratory echocardiographic and computed tomography evaluation could have impacted the assessment of baseline and procedural results. Fourth, implantation depth was not assessed in the current study. Fifth, data on simultaneous haemodynamic measurements were not available. Also, the incidence of predicted PPM was not assessed in this study. Finally, we need to acknowledge the lack of power in assessing differences in all-cause mortality deriving from the non-uniform distribution of patients among groups, although it should be recognised that our retrospective cohort study provides a relevant real-world picture of the practice at 16 high-volume valve centres.
Conclusions
The TAVI-SMALL 2 multicentre observational retrospective registry, including patients with aortic stenosis and small aortic annuli undergoing transfemoral TAVI, suggests that the implantation of SAV and SEV yields lower mean aortic valve gradients and protects from the development of severe PPM when compared to IAV and BEV, respectively, at the expense of higher rates of PVL. Also, PPI was more common after SEV than BEV implantation. Randomised trials assessing the long-term prognostic relevance of the type of THV implanted in small aortic annuli are eagerly awaited.
Impact on daily practice
The TAVI-SMALL 2 international multicentre registry is the largest to date to compare the performance of contemporary transcatheter valves in patients with aortic stenosis and small annuli undergoing TAVI. SAV and SEV yielded lower mean aortic valve gradients and incidence of severe PPM when compared to IAV and BEV, respectively, at the expense of higher rates of paravalvular leak. Permanent pacemaker implantation was more common after SEV than BEV implantation. This study supports the implantation of SAV for superior forward flow haemodynamics in patients with small annuli. The long-term relevance of PPM after TAVI will need to be addressed in larger randomised studies.
Guest editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg – Bad Krozingen, Bad Krozingen, Germany.
Conflict of interest statement
M. Barbanti is a consultant for Medtronic, Boston Scientific, and Edwards Lifesciences. R. Teles has received a received a research grant (to the institution) from Abbott. M. Adamo discloses speaker fees from Abbott and Medtronic. M. Taramasso discloses consultant or consultancy fees from Abbott Vascular, Edwards Lifesciences, Boston Scientific, Medtronic, Shenqi Medical, VentriMend, Simulands, Occlufit, MTEx, MEDIRA, and HI-D Imaging; and serves on the advisory board of Abbott. G. Stefanini has received a research grant (to the institution) from Boston Scientific; and has received speaking and consulting fees from Abbott Vascular, Boston Scientific, and Pfizer/BMS. W-K. Kim is a proctor for Boston Scientific, Meril Life Sciences, and Abbott; and has received speaking fees from Boston Scientific, Abbott, Medtronic, Edwards Lifesciences, Meril Life, and Shockwave Medical. F. Maisano discloses grant and/or research institutional support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific, NVT, and Terumo; consulting fees, personal and institutional honoraria from Abbott, Medtronic, Edwards Lifesciences, Xeltis, Cardiovalve, Occlufit, Simulands, and Mtex; royalty income/IP rights from Edwards Lifesciences; a shareholder position (including share options) at Cardiogard, Cardiovalve, Magenta, SwissVortex, Transseptal Solutions, 4Tech, and Perifect. N.M. Van Mieghem has received institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, Abiomed, PulseCath BV, Daiichi Sankyo, and Biotronik. B. Reimers has received speaking honoraria from Boston Scientific. A. Latib serves on the advisory boards of Medtronic, Edwards Lifesciences, Boston Scientific, Philips, and Abbott. The other authors have no conflicts of interest to declare, relevant to the contents of this paper. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Supplementary data
To read the full content of this article, please download the PDF.

