Abstract
Background: A small aortic annulus (SAA) is a risk factor for prosthesis-patient mismatch (PPM) in patients undergoing surgical or transcatheter aortic valve implantation (TAVI). Data regarding TAVI in patients with extra-SAA are scarce.
Aims: The aim of this study was to analyse the safety and efficacy of TAVI in patients with extra-SAA.
Methods: A multicentre registry study including patients with extra-SAA (defined as an aortic annulus area <280 mm2 and/or perimeter <60 mm) undergoing TAVI was established. Primary efficacy and safety endpoints were defined as device success and early safety at 30 days, respectively, using the Valve Academic Research Consortium-3 criteria, and were analysed according to valve type: self-expanding (SEV) versus balloon-expandable (BEV).
Results: A total of 150 patients were included, of which 139 (92.7%) were women, and 110 (73.3%) received an SEV. Intraprocedural technical success was 91.3%, with a higher rate in patients receiving an SEV (96.4% vs 77.5% with BEV; p=0.001). Overall, 30-day device success was 81.3%, (85.5% with SEV vs 70.0% with BEV; p=0.032). The primary safety endpoint occurred in 72.0% of patients (with no difference between groups; p=0.118). Severe PPM occurred in 12% (9.0% with SEV and 24.0% with BEV; p=0.039), with no impact on all-cause mortality, cardiovascular mortality, or heart failure readmission at 2-year follow-up.
Conclusions: TAVI is a safe and feasible treatment in patients with extra-SAA with a high rate of technical success. The use of SEV was associated with a lower rate of intraprocedural complications, higher device success at 30 days and better haemodynamic outcomes compared to BEV.
Introduction
Transcatheter and surgical aortic valve replacement (TAVI and SAVR) are both effective treatments for severe symptomatic aortic stenosis. With technical improvements in TAVI and the expansion of clinical indications to a broad spectrum of risk profiles12, individual anatomical characteristics are of paramount importance when ascertaining the optimal individualised treatment strategy and determining the ideal type of transcatheter bioprosthetic aortic valve34. Small aortic annuli (SAA) represent an anatomical challenge for AVR and have been associated with poorer outcomes after SAVR, with increased mortality, ischaemic cardiovascular events, and prosthesis-patient mismatch (PPM)5. The prevalence of SAA (defined as prosthesis size ≤21 mm in patients undergoing SAVR) ranges between 22% and 34% in the United States and Northern Europe, with a strong preponderance in female patients678.
TAVI has demonstrated better haemodynamic performance and a lower rate of PPM compared with SAVR, specifically in patients with SAA910. In TAVI, self-expanding valves (SEV) have been associated with superior haemodynamic results as compared with balloon-expandable valves (BEV)11121314. However, the American Guideline for the Management of Patients with Valvular Heart Disease favours SAVR with prior surgical annulus enlargement in patients with SAA to allow for the placement of a larger prosthesis1, although, this technique is not frequently performed. Clinical real-world data regarding patients with extra-SAA − annuli dimensions close to or below the manufacturer’s recommended lower range for transcatheter heart valves (THV) − undergoing TAVI are limited.
The aim of this study was to analyse and compare periprocedural complications, haemodynamic performance, and midterm clinical outcomes in patients with severe native aortic stenosis and extra-SAA undergoing TAVI with either SEV or BEV.
Methods
Consecutive patients with severe aortic stenosis and extra-SAA undergoing TAVI were included from 24 centres in Europe, Canada, and Israel. Eligibility for TAVI and postprocedural management were determined by the Heart Team at each centre. THV type, size, and implantation technique were performed at the operators’ discretion. Data were prospectively collected in a dedicated database, in accordance with the local ethics committee of each participating centre, and all patients provided signed informed consent for the procedures.
Inclusion criteria were patients treated with TAVI for severe native aortic stenosis with extra-SAA, defined as an aortic annulus perimeter ≤60 mm or an aortic annulus area ≤280 mm2 as determined by computed tomography (CT). These thresholds were based on the lower limit stated in the information for use (IFU) of the smaller size Portico/Navitor (Abbott Vascular) and SAPIEN (Edwards Lifesciences) devices. The IFU of the CoreValve Evolut (Medtronic) recommends larger oversizing (13-29%), so the lower limit of the 23 mm device is smaller (perimeter of 56.5 mm). Portico/Navitor and SAPIEN cutoff points were used instead of Evolut to obtain a more inclusive sample so that different degrees of oversizing could be assessed. Exclusion criteria were patients requiring valve-in-valve procedures and patients with no implanted THVs. No limits were set with respect to the type of THV implanted.
Primary efficacy and safety endpoints were defined as device success and early safety at 30 days, respectively, according to the Valve Academic Research Consortium-3 (VARC-3) definitions15. Device success at 30 days was defined as intraprocedural technical success, freedom from 30-day mortality, freedom from 30-day surgery or intervention related to the device, access or cardiac structural complication, and intended THV performance (peak velocity <3 m/s, Doppler velocity index ≥0.25, mean gradient <20 mmHg, and less than moderate aortic regurgitation [AR]). Early safety at 30 days was defined as freedom from all-cause mortality; stroke; VARC-3 type 2-4 bleeding; major vascular, access-related, or cardiac structural complications; acute kidney injury stage 3 or 4; moderate or severe AR; new permanent pacemaker; and surgery or intervention related to the device. Intraprocedural technical success was defined as freedom from mortality, successful access, delivery of a single THV with correct positioning and retrieval of the delivery system, freedom from surgery or intervention related to the device or to a vascular complication, and freedom from structural cardiac complications.
The degree of oversizing was calculated as ([nominal prosthesis perimeter/annulus perimeter] −1)×100% for SEV and as ([nominal prosthesis diameter/annulus area-derived diameter] −1)×100% for BEV1617. If BEV were implanted with less cc than the nominal volume, the prosthesis area was calculated as (nominal device area*filling volume)/nominal volume, and the degree of oversizing was estimated using the calculated prosthesis area-derived diameter. The echocardiographic outcomes were defined according to VARC-3 criteria. The effective orifice area (EOA) was acquired at discharge or within 1 month after the procedure. Body mass index-specific cutoffs were used to determine the presence of PPM. In patients with a body mass index <30 kg/m2, PPM was defined as none or mild if the indexed EOA (iEOA) was>0.85 cm2/m2, moderate if the iEOA was 0.85-0.66 cm2/m2 and severe if the iEOA was ≤0.65 cm2/m2. In patients with a body mass index ≥30 kg/m2, PPM was defined as none or mild if the iEOA was>0.70 cm2/m2, moderate if the iEOA was 0.56-0.70 cm2/m2 and severe if the iEOA was ≤0.55 cm2/m2. Clinical follow-up was performed in an outpatient clinic setting, according to local protocols. Data were collected in a dedicated electronic database by experienced physicians at each centre, then merged and retrospectively analysed in the coordinating centre.
STATISTICAL ANALYSIS
Categorical variables were summarised as n (percentage) and compared between groups using a Chi-square test or Fisher’s exact test, as appropriate. Continuous variables were summarised as mean (standard deviation [SD]) or median (interquartile range [IQR]: 25-75th percentile) and compared using a 2-sided Student t-test or Mann-Whitney U test according to their distribution. Assessment of normality for continuous data was performed using the Shapiro-Wilk test. A propensity score-matched cohort was created with a 1:1 ratio of SEV and BEV patients using a “nearest neighbour” match without replacement. A calliper of <0.2x the SD of the logistic score was applied. The balance between groups was visually assessed before and after the matching with the smoothed kernel-density plots of the logistic score (Supplementary Figure 1). Standardised mean differences (SMD) were calculated for covariates in order to assess for potential imbalances between both cohorts. Survival analyses were performed using a Kaplan-Meier survival function. Survival comparisons were performed using a log-rank test. P-values less than 0.05 were regarded as statistically significant. Multivariable Cox regression was performed to evaluate the predictors of 2-year mortality or heart failure readmission. All data were analysed using Stata, version 14 (StataCorp).
Results
Among a total of 150 patients eligible for the study, 110 (73.3%) patients underwent TAVI with an SEV and 40 (26.7%) with a BEV. Age, peripheral vascular disease, baseline mean aortic gradient, previous stroke, and bicuspid aortic valve were included in the propensity score analysis, resulting in 70 matched patients (1:1) with SEV and BEV. Baseline clinical characteristics and imaging data of the overall population and the matched cohort are summarised in Table 1. The median age of the patients was 83 (IQR 78-87), 92.7% were women, with a median body surface area (BSA) of 1.60 (IQR 1.49-1.72) m2. The median aortic annulus perimeter was 59.1 (IQR 58.0-60.0) mm (minimum 51.0 mm) with an aortic area of 271 (IQR 258-280) mm2 (minimum 196 mm2). Before matching, patients undergoing TAVI with BEV were older compared to those with SEV and had higher transvalvular gradients at baseline echocardiography (Table 1).
Procedural details are showed in Table 2. Transfemoral access was used in 95.3% of the cases. In this cohort of patients with extra-SAA, the majority (72.7%) were treated with a 23 mm SEV. The Evolut R or PRO were the most commonly used SEV (60.0% of patients), followed by the Portico/Navitor, which was used in 17.3% of patients. Among those receiving BEV, either the SAPIEN 3 or the SAPIEN 3 Ultra was implanted in 52.5%. As expected, the mean prosthesis size and oversizing were significantly higher in those with SEV compared with BEV (23.6±1.2 mm vs 21.5±1.5 mm; p<0.001 and 24.6 [IQR 20.8-29.0] % vs 14.3 [IQR 7.7-24.4] %; p<0.001, respectively). A total of 20 patients received a 23 mm BEV, with an underfilled balloon in 6 (30%) patients (1cc less in 4 patients and 1.5cc less in 2 patients), decreasing the oversizing from 25.0 (IQR 23.8-26.4) % to 14.3 (IQR 12.2-17.4) % in patients with nominal versus underfilled balloons, respectively. The oversizing was 7.9 (IQR 5.9-10.9) % in patients receiving a nominal 20 mm BEV, compared to â1.5 (IQR â3.1-0.3) % oversizing in 4 (20%) cases with 1cc less in the balloon.
Periprocedural and in-hospital outcomes are presented in Table 3 and Figure 1. Technical failure occurred in 13 (8.7%) patients, being more frequent in the BEV group in the overall cohort (9 [22.5%] vs 4 [3.6%]; p=0.001), and in the matched cohort (7 [20.0%] vs 1 [2.9%]; p=0.055). There were no cases of aortic annulus rupture, but intra-annular aortic-contained haematoma occurred in 2 (1.3%) patients who were treated with a 23 mm BEV. Both patients were managed conservatively and survived to discharge. One patient was readmitted within 1 week post-TAVI due to severe paravalvular leak after reabsorption of the haematoma. After optimisation of medical treatment, the patient survived 7.7 years and died from a non-cardiovascular cause. The second patient did not present any adverse outcomes after 2-year follow-up. Coronary occlusion occurred in 3 (2.0%) patients after the implantation of a 26 mm Evolut R, a 23 mm SAPIEN XT and a 23 mm SAPIEN 3 THV. The first two were ostial right and ostial left coronary obstructions, with valve oversizing of 33.9% and 23.8%, respectively, managed with percutaneous coronary stenting, without further in-hospital complications. The third was a left anterior descending artery mid-segment occlusion due to embolisation during valve guidewire crossing, managed with thromboaspiration.
There were 2 cases of valve migration. One patient experienced ventricular migration of a SAPIEN XT valve, requiring conversion to open heart surgery. The second case needed a second valve (SAPIEN 3) due to the migration of the Evolut R to a supra-annular position. Cardiac tamponade occurred in 5 (3.3%) patients, 1 (0.9%) in the SEV group and 4 (10%) in the BEV group (p=0.001), one of the patients from the BEV group required conversion to open heart surgery. Causes of cardiac tamponade were left ventricle perforation by the stiff wire (n=2) and right ventricle perforation by the temporary pacemaker lead (n=3). Intraprocedural death occurred in 1 case due to a vascular complication in the abdominal aorta.
The primary efficacy endpoint (device success) at 30 days was observed in 81.3% of the patients, more commonly achieved in the SEV group (85.5%) compared to the BEV group (70.0%); p=0.032 (Figure 1, Central illustration). The primary safety endpoint occurred in 72.0% (SEV 75.5% vs BEV 62.5%; p=0.118), with a high rate of pacemaker implantation (19.4%), without differences between groups (p=0.913). The primary efficacy endpoint in the matched cohort continued to be higher in the SEV group compared to the BEV group (91.4% vs 68.6%; p=0.034), with a similar rate of the primary safety endpoint (71.4% vs 65.7%; p=0.607) (Table 3, Supplementary Figure 2).
In-hospital mortality occurred in 3 (2.0%) patients (1 due to life-threatening bleeding from a vascular complication in the iliac artery, 1 due to a respiratory cause, and the previously described intraprocedural death). Major vascular in-hospital complications occurred in 7.3% and type 2 to 4 VARC-3 bleeding complications occurred in 14.0%.
Mild AR was observed in 41 patients (27.7%) and moderate AR in 8 (5.4%), with no cases of severe AR. Overall, the peak and mean gradients at discharge were 19 (IQR 14-26) mmHg and 11 (IQR 8-15) mmHg, respectively. The median EOA was 1.4 (IQR 1.2-1.7) cm2 (index EOA: 0.9 [IQR 0.7-1.0] cm2/m2) (Figure 2). Patients with an SEV, compared to a BEV, showed lower transaortic valve peak and mean gradients (18 [IQR 13-25] vs 25 [IQR 18-32] mmHg; p=0.026 and 10 [IQR 8-13] vs 14 [IQR 9-19]; p=0.010, respectively) and higher EOA and iEOA (1.50 [IQR 1.30-1.70] vs 1.12 [IQR 1.00-1.34] cm2; p<0.001 and 0.93 [IQR 0.77-1.04] vs 0.73 [IQR 0.65-0.81] cm2/m2; p<0.001, respectively) (Table 4, Figure 2). A mean transprosthesis gradient ≥20 mmHg occurred in 8.1% patients (4.6% in the SEV and 18.0% in the BEV group; p=0.008). The overall incidence of severe PPM was 12.0%, with significant differences between the SEV and BEV groups (9.0% vs 24.0% respectively; p=0.039) (Central illustration). A similar difference in the haemodynamic valve performance between SEV and BEV was observed in the matched cohort (Table 4, Supplementary Figure 3). The haemodynamic results between the 23 mm Evolut R (n=28) and the 23 mm Evolut PRO (n=20) were compared to assess the potential impact of the sealing skirt from the latest generation of the Evolut platform, with no significant differences observed in valve performance (mean gradient: 11 [IQR 10-16] vs 10 [IQR 9-17] mmHg; p=0.720; EOA: 1.4 [IQR 1.3-1.6] vs 1.6 [IQR 1.3-1.7] cm2; p=0.196; and moderate or severe PPM: 38.5 vs 27.8%; p=0.462, respectively). Otherwise, in the overall cohort, THV oversizing was not significantly associated with the rate of severe PPM (25.2 [IQR 9.9-28.0] % vs 27.2 [IQR 20.4-28.0] % in patients with and without severe PPM; p=0.885).
Follow-up was available in all cases, with a median time of 28.3 months [IQR 12.6-38.4]. At 1-year follow-up, New York Heart Association (NYHA) Functional Class I or II was observed in 89.8%. Two-year all-cause mortality was 11.5% (SEV 13.0% vs BEV 7.9%; p=0.445) and cardiovascular mortality was 5.2% (SEV 5.0% vs BEV 5.5%; p=0.986). Heart failure admission at 2 years occurred in 8.0% (8.4% in BEV group and 8.0% in SEV; p=0.812) (Figure 3). Severe PPM was not associated with a higher risk of all-cause mortality, cardiovascular mortality, or heart failure admission at 2 years (hazard ratio [HR] 1.6, 95% confidence interval [CI]: 0.3-7.2; p=0.554; HR 1.5, 95% CI: 0.2-12.8; p=0.716; and HR 2.0, 95% CI: 0.4-9.5; p=0.395, respectively). Furthermore, neither severe PPM nor a mean transprosthesis gradient >20 mmHg were associated with the combined endpoint of mortality or heart failure admission at 2 years (HR 1.4, 95% CI: 0.4-4.9; p=0.591 and HR 0.5, 95% CI: 0.1-3.7; p=0.501, respectively). The identified predictors for the combined endpoint were bleeding complications type 3 and 4, acute kidney injury, and new permanent pacemaker implantation (Supplementary Table 1).
Table 1. Baseline characteristics according to type of THV.
| Overall cohort N=150 | Overall cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| SEV N=110 | BEV N=40 | p-value | SEV N=35 | BEV N=35 | p-value | ||
| Baseline clinical characteristics | |||||||
| Age, years | 83 [78-87] | 82 [77-86] | 86 [79-89] | 0.018 | 86 [79-89] | 86 [79-89] | 0.809 |
| Female sex | 139 (92.7) | 100 (90.9) | 39 (97.5) | 0.289 | 35 (100) | 31 (88.6) | 0.114 |
| Weight, kg | 62 [54-68] | 62 [55-68] | 59 [50-67] | 0.233 | 64 [56-68] | 55 [50-65] | 0.076 |
| Height, cm | 153 [147-158] | 153 [147-159] | 152 [145-156] | 0.256 | 154 [147-157] | 150 [144-157] | 0.246 |
| Body mass index, kg/m2 | 26.0 [23.4-29.0] | 26.0 [23.4-29.0] | 26.1 [22.0-20.0] | 0.976 | 27.0 [23.5-30.4] | 25.9 [21.5-28.9] | 0.391 |
| Body surface area, m2 | 1.60 [1.49-1.72] | 1.61 [1.53-1.71] | 1.57 [1.44-1.72] | 0.175 | 1.6 [1.6-1.7] | 1.6 [1.4-1.7] | 0.076 |
| Diabetes | 49 (32.7) | 36 (32.7) | 13 (32.5) | 0.979 | 11 (31.4) | 11 (31.4) | 0.999 |
| Hypertension | 126 (84.0) | 92 (83.6) | 34 (85.0) | 0.840 | 32 (91.4) | 29 (82.9) | 0.477 |
| Coronary artery disease | 45 (30.0) | 36 (32.7) | 9 (22.5) | 0.227 | 8 (22.9) | 8 (22.9) | 0.999 |
| Previous CABG | 8 (5.3) | 8 (7.3) | 0 | 0.110 | 1 (2.9) | 0 | 0.999 |
| Atrial fibrillation | 44 (29.5) | 34 (31.2) | 10 (25.0) | 0.463 | 12 (34.3) | 9 (25.7) | 0.434 |
| COPD | 21 (14.0) | 16 (14.6) | 5 (12.5) | 0.750 | 5 (14.3) | 5 (14.3) | 0.999 |
| Previous stroke | 15 (10.0) | 14 (12.7) | 1 (2.5) | 0.072 | 2 (5.7) | 1 (2.9) | 0.999 |
| Peripheral vascular disease | 16 (10.7) | 15 (13.6) | 1 (2.5) | 0.070 | 0 (0) | 1 (2.9) | 0.999 |
| EuroSCORE II | 3.7 [2.3-5.6] | 3.7 [2.4-5.4] | 3.6 [2.2-5.8] | 0.961 | 4.4 [2.3-5.3] | 3.5 [2.2-5.8] | 0.880 |
| STS score | 4.6 [3.1-7.0] | 4.4 [3.0-6.0] | 5.2 [3.6-7.4] | 0.323 | 5.1 [3.1-8.5] | 4.4 [2.3-5.3] | 0.588 |
| Frailty | 58/125 (46.4) | 42/86 (48.8) | 16/39 (41.0) | 0.417 | 13 (37.1) | 13 (37.1) | 0.999 |
| Baseline echocardiographic parameters | |||||||
| LVEF, % | 61 [60-68] | 60 [60-68] | 61 [60-69] | 0.551 | 65 [60-69] | 61 [60-69] | 0.344 |
| Mean aortic gradient, mmHg | 49 [41-61] | 47 [41-60] | 53 [44-62] | 0.095 | 54 [45-64] | 53 [44-62] | 0.819 |
| Peak aortic gradient, mmHg | 80 [68-97] | 78 [68-94] | 89 [71-105] | 0.054 | 84 [74-91] | 87 [71-105] | 0.458 |
| Aortic valve area, cm2 | 0.65 [0.58-0.78] | 0.66 [0.60-0.80] | 0.60 [0.54-0.70] | 0.156 | 0.5 [0.5-0.8] | 0.6 [0.6-0.7] | 0.823 |
| Bicuspid aortic valve | 4/133 (3.0) | 1/95 (1.1) | 3/38 (7.9) | 0.070 | 1 (2.9) | 2 (5.7) | 0.999 |
| Moderate-to-severe AR | 38/130 (29.2) | 30/94 (31.9) | 8/36 (22.2) | 0.277 | 12/35 (34.3) | 7 (21.2) | 0.285 |
| Moderate-to-severe MR | 41 (27.9) | 28 (26.2) | 13 (32.5) | 0.446 | 12 (34.3) | 12 (34.3) | 0.999 |
| Baseline computed tomography data | |||||||
| AA perimeter, mm | 59.1 [58.0-60.0] | 59.0 [58.0-60.0] | 59.9 [58.7-61.0] | 0.067 | 59.2 [57.7-60] | 60 [58.9-60] | 0.044 |
| AA area, mm2 | 270 [259-280] | 271 [258-280] | 266 [260-277] | 0.329 | 270 [260-280] | 272 [258-280] | 0.517 |
| AA max diameter, mm | 21.0 [19.5-22.0] | 20.7 [19.2-22.0] | 21.0 [20.0-21.6] | 0.380 | 21.0 [19.4-22.0] | 21.0 [19.0-21.6] | 0.870 |
| AA min diameter, mm | 16.3 [15.5-17.1] | 16.4 [15.5-17.1] | 16.0 [15.3-17.0] | 0.696 | 16.7 [15.5-17.1] | 16.4 [15.8-17.0] | 0.860 |
| Perimeter-derived diameter, mm | 18.8 [18.5-19.1] | 18.8 [18.5-19.1] | 19.1 [18.7-19.4] | 0.067 | 18.8 [18.4-19.1] | 19.1 [18.7-19.4] | 0.044 |
| Area-derived diameter, mm | 18.5 [18.2-18.9] | 18.6 [18.1-18.9] | 18.4 [18.2-18.8] | 0.329 | 18.6 [18.1-18.9] | 18.5 [18.2-18.8] | 0.517 |
| Sinus of Valsalva diameter, mm | 26.4 [25.0-28.0] | 26.2 [24.8-27.5] | 27.1 [25.0-28.5] | 0.163 | 26.4 [24.9-27.8] | 27 [25.3-28.5] | 0.123 |
| Left coronary height, mm | 11.5 [10.0-13.0] | 11.4 [10.0-13.0] | 12 [9.9-13.6] | 0.431 | 11.4 [11-12.3] | 12 [10.2-13.6] | 0.361 |
| Right coronary height, mm | 12.0 [10.0-14.0] | 12.0 [10.0-13.7] | 12.2 [10.0-14.8] | 0.690 | 12.0 [10.0-14.0] | 12.2 [10.0-14.8] | 0.782 |
| Eccentricity index | 0.20 [0.14- 0.27] | 0.19 [0.13-0.27] | 0.24 [0.15-0.29] | 0.171 | 0.19 [0.11-0.27] | 0.23 [0.14-0.27] | 0.611 |
| Values are expressed as median [IQR], n (%) or n/N (%). AA: aortic annulus; AR: aortic regurgitation; BEV: balloon-expandable valve; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; EuroSCORE: European System for Cardiac Operative Risk Evaluation; IQR: interquartile range; LVEF: left ventricular ejection fraction; MI: myocardial infarction; MR: mitral regurgitation; SEV: self-expanding valve; STS: Society of Thoracic Surgeons; THV: transcatheter heart valve | |||||||
Table 2. Procedural details according to type of THV.
| Procedural details | Overall cohort N=150 | Overall cohort | Matched cohort | ||||
|---|---|---|---|---|---|---|---|
| SEV N=110 | BEV N=40 | p-value | SEV N=35 | BEV N=35 | p-value | ||
| Transfemoral access | 143 (95.3) | 103 (93.6) | 40 (100) | 0.611 | 34 (97.1) | 35 (100) | 0.999 |
| Transapical access | 1 (0.7) | 1 (0.9) | 0 | 0 | 0 | ||
| Transaxillary access | 3 (2.0) | 3 (2.7) | 0 | 1 (2.9) | 0 | ||
| Transcarotid access | 3 (2.0) | 3 (2.7) | 0 | 0 | 0 | ||
| Transcatheter heart valve | |||||||
| SAPIEN and SAPIEN XT (Edwards Lifesciences) | 18 (12.0) | 0 | 18 (45.0) | 0 | 16 (45.7) | ||
| SAPIEN 3 or SAPIEN 3 Ultra (Edwards Lifesciences) | 21 (14.0) | 0 | 21 (52.5) | 0 | 19 (54.3) | ||
| CoreValve (Medtronic) | 10 (6.7) | 10 (9.1) | 0 | 7 (20.0) | 0 | ||
| Evolut R (Medtronic) | 36 (24.0) | 36 (32.7) | 0 | 14 (40.0) | 0 | ||
| Evolut PRO (Medtronic) | 30 (20.0) | 30 (27.3) | 0 | 5 (14.3) | 0 | ||
| Portico or Navitor (Abbott) | 19 (12.7) | 19 (17.3) | 0 | 4 (11.4) | 0 | ||
| ACURATE neo (Boston Scientific) | 11 (7.3) | 11 (10.0) | 0 | 3 (8.6) | 0 | ||
| Other | 5 (3.3) | 4 (3.6) | 1 (2.5) | 2 (5.7) | 0 | ||
| Prosthesis size | |||||||
| 20 mm | 20 (13.3) | 0 | 20 (50.0) | <0.001 | 0 | 17 (48.6) | <0.001 |
| 23 mm | 109 (72.7) | 89 (80.9) | 20 (50.0) | 28 (80.0) | 18 (51.4) | ||
| 26 mm | 21 (14.0) | 21 (19.1) | 0 | 7 (20.0) | 0 | ||
| Prosthesis size, mm | 23.0 (1.6) | 23.6 (1.2) | 21.5 (1.5) | <0.001 | 23.6 (1.2) | 21.5 (1.5) | <0.001 |
| Oversizing, % | 22.6 [20.2-27.0] | 24.6 [20.8-29.0] | 14.3 [7.7-24.4] | <0.001 | 24.6 [20.4-28.1] | 14.4 [7.9-24.3] | <0.001 |
| Prior balloon valvuloplasty | 68/142 (47.9) | 51/102 (50.0) | 17/40 (42.5) | 0.421 | 22 (67.7) | 14 (40.0) | 0.040 |
| Balloon post-dilation | 34 (22.8) | 26 (23.9) | 8 (20.0) | 0.619 | 12 (34.3) | 8 (22.9) | 0.290 |
| Contrast volume, ml | 133 [105-182] | 148 [110-182] | 113 [68-167] | 0.054 | 153 [105-196] | 132 [74-170] | 0.220 |
| Procedure time, min | 95 [65-120] | 95 [64-120] | 93 [70-122] | 0.858 | 98 [72-128] | 93 [59-123] | 0.774 |
| Values are expressed as mean (SD), median [IQR], n (%) or n/N (%). BEV: balloon-expandable valve; IQR: interquartile range; SD: standard deviation; SEV: self-expanding valve; THV: transcatheter heart valve | |||||||
Table 3. In-hospital complications and clinical outcomes in the overall cohort and matched cohort according to the type of THV.
| Overall cohort N=150 | Overall cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| SEV N=110 | BEV N=40 | p-value | SEV N=35 | BEV N=35 | p-value | ||
| INTRAPROCEDURAL COMPLICATIONS | |||||||
| Intraprocedural death | 1 (0.7) | 0 | 1 (2.5) | 0.267 | 0 | 1 (2.9) | 0.999 |
| Aortic annulus rupture | 0 | 0 | 0 | - | 0 | 0 | |
| Aortic annulus contained haematoma | 2 (1.3) | 0 | 2 (5.0) | 0.070 | 0 | 1 (2.9) | 0.999 |
| Coronary occlusion | 3 (2.0) | 1 (0.9) | 2 (5.0) | 0.174 | 0 | 2 (5.7) | 0.493 |
| Implantation of multiple (>1) valves | 1 (0.7) | 1 (0.9) | 0 | 0.999 | 0 | 0 | |
| Conversion to open heart surgery | 2 (1.3) | 0 | 2 (5.0) | 0.070 | 0 | 1 (2.9) | 0.999 |
| Cardiac tamponade | 5 (3.3) | 1 (0.9) | 4 (10.0) | 0.018 | 1 (2.9) | 3 (8.6) | 0.614 |
| Technical success | 137 (91.3) | 106 (96.4) | 31 (77.5) | 0.001 | 34 (97.1) | 28 (80.0) | 0.055 |
| IN-HOSPITAL CLINICAL OUTCOMES | |||||||
| In-hospital mortality | 3 (2.0) | 2 (1.8) | 1 (2.5) | 0.999 | 0 | 1 (2.9) | 0.999 |
| Cerebrovascular events | 3 (2.0) | 2 (1.8) | 1 (2.5) | 0.999 | 0 | 1 (2.9) | 0.999 |
| Transient ischaemic attack | 1 (0.7) | 1 (0.9) | 0 | 0.999 | 0 | 0 | - |
| Stroke | 2 (1.3) | 1 (0.9) | 1 (2.5) | 0.464 | 0 | 1 (2.9) | 0.999 |
| Vascular complications | |||||||
| Major vascular complication | 11 (7.3) | 8 (7.3) | 3 (7.5) | 0.999 | 2 (5.7) | 2 (5.7) | 0.999 |
| Minor vascular complication | 22 (14.7) | 17 (15.5) | 5 (12.5) | 0.651 | 7 (20.0) | 5 (14.3) | 0.752 |
| Bleeding complications | |||||||
| Type 1 | 25 (16.7) | 19 (17.3) | 6 (15.0) | 0.741 | 9 (25.1) | 6 (17.1) | 0.561 |
| Type 2 | 12 (8.0) | 8 (7.3) | 4 (10.0) | 0.734 | 4 (11.4) | 4 (11.4) | 0.999 |
| Type 3 | 7 (4.7) | 3 (2.7) | 4 (10.0) | 0.082 | 2 (5.7) | 3 (8.6) | 0.999 |
| Type 4 | 2 (1.3) | 1 (0.9) | 1 (2.5) | 0.464 | 0 | 1 (2.9) | 0.999 |
| Acute kidney injury | |||||||
| Stage 1 | 16 (10.7) | 11 (10.0) | 5 (12.5) | 0.909 | 2 (5.7) | 5 (14.3) | 0.265 |
| Stage 2 | 2 (1.3) | 2 (1.8) | 0 | 2 (5.7) | 0 | ||
| Stage 3 or 4 | 1 (0.7) | 1 (0.9) | 0 | 0 | 0 | - | |
| New onset atrial fibrillation | 5 (3.4) | 3 (2.8) | 2 (5.1) | 0.610 | 12 (34.3) | 9 (25.7) | 0.434 |
| New permanent pacemaker implantation* | 25/129 (19.4) | 18/94 (19.2) | 7/35 (20.0) | 0.913 | 7 (23.3) | 5 (16.7) | 0.519 |
| Device success at 30 days | 122 (81.3) | 94 (85.5) | 28 (70.0) | 0.032 | 32 (91.4) | 24 (68.6) | 0.034 |
| Procedural safety at 30 days | 108 (72.0) | 83 (75.5) | 25 (62.5) | 0.118 | 25 (71.4) | 23 (65.7) | 0.607 |
| Length of hospital stay, days | 5 [3-7] | 5 [3-7] | 4 [3-6] | 0.966 | 6 [4-10] | 4 [3-6] | 0.050 |
| Values are expressed as n (%), n/N (%) or median [IQR]. *Excluding patients with previous pacemaker. BEV: balloon-expandable valve; IQR: interquartile range; SEV: self-expanding valve; THV: transcatheter heart valve | |||||||
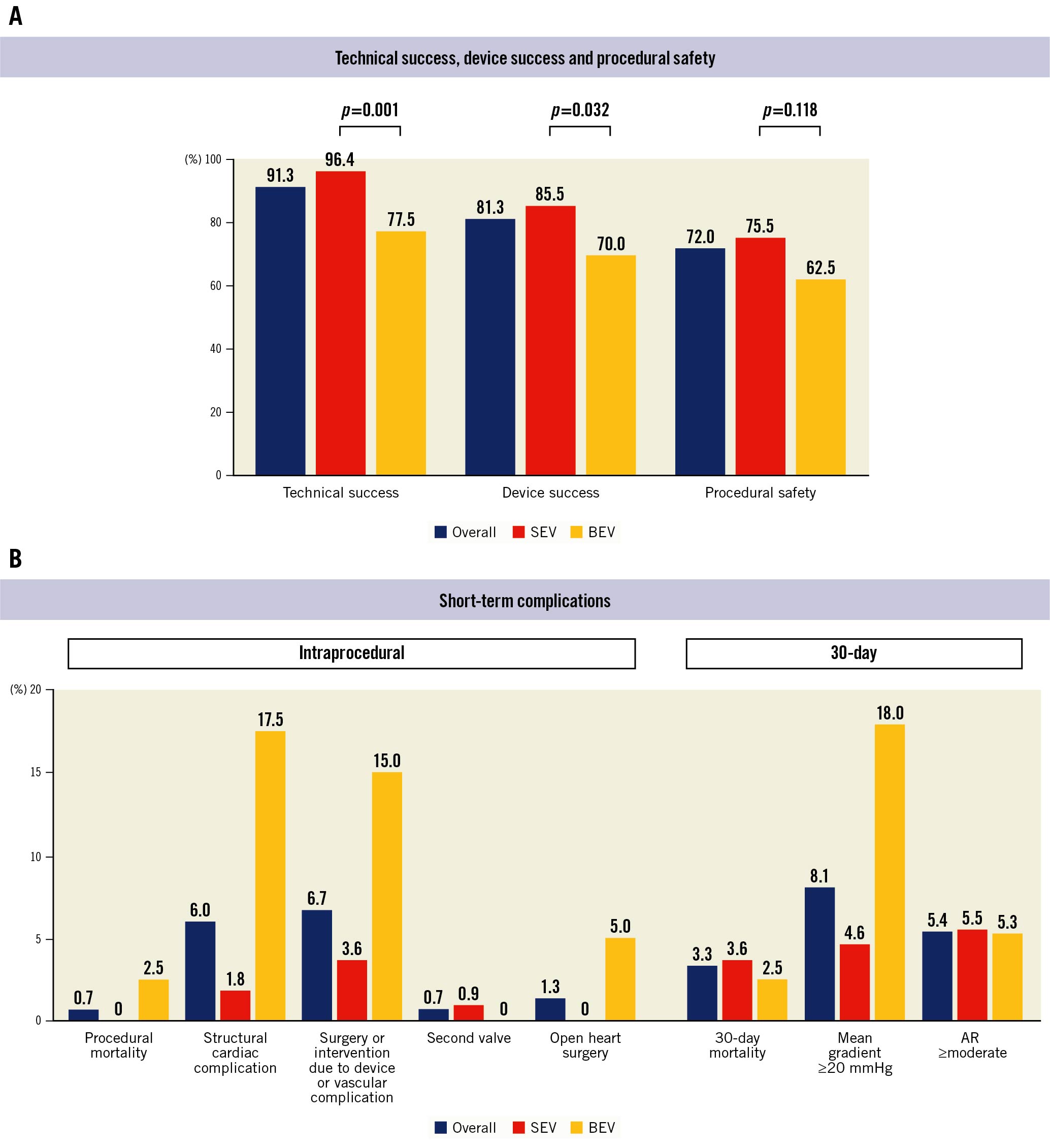
Figure 1. Intraprocedural and 30-day outcomes of transcatheter aortic valve replacement in patients with extra-small aortic annuli. A) Incidence of technical success, device success, and procedural safety in the overall cohort, according to self-expanding (SEV) or balloon-expandable (BEV) valves. B) Incidence of periprocedural and 30-day complications in the overall cohort, according to self-expanding (SEV) or balloon-expandable (BEV) valves. AR: aortic regurgitation
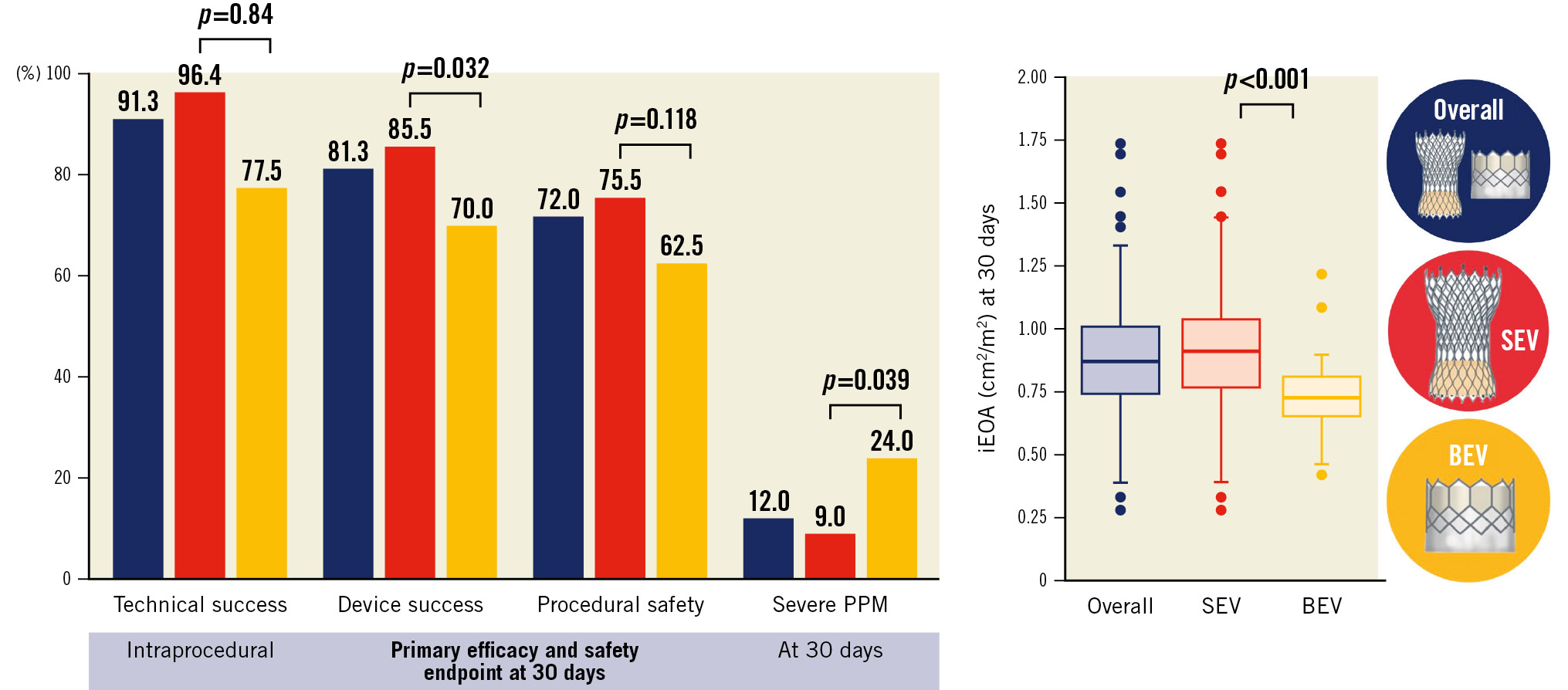
Central illustration. Safety, efficacy, and haemodynamic results of transcatheter aortic valve replacement in patients with extra-small aortic annuli. A) Overall rate of technical success, device success, procedural safety, and severe prosthesis-patient mismatch, according to the type of transcatheter heart valve. B) Indexed effective orifice area after transcatheter aortic valve replacement. BEV: balloon-expandable valve; iEOA: indexed effective orifice area; PPM: prosthesis-patient mismatch; SEV: self-expanding valve
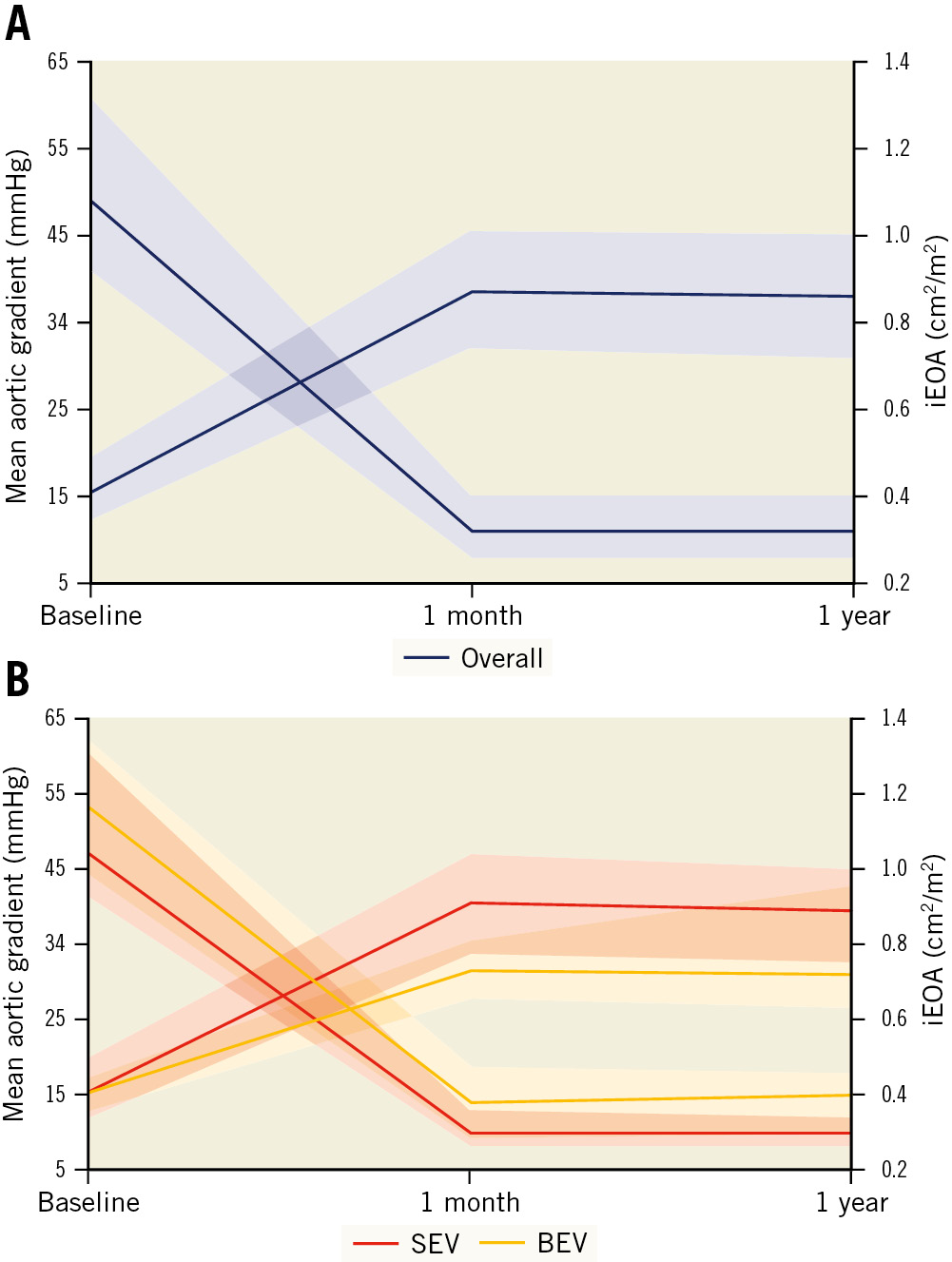
Figure 2. Haemodynamic results after transcatheter aortic valve replacement in patients with extra-small aortic annuli. A) Overall valve haemodynamic results. B) According to valve type. BEV: balloon-expandable valve; iEOA: indexed effective orifice area; SEV: self-expanding valve
Table 4. Haemodynamic outcomes.
| Overall cohort N=150 | Overall cohort | Matched cohort | |||||
|---|---|---|---|---|---|---|---|
| SEV N=110 | BEV N=40 | p-value | SEV N=35 | BEV N=35 | p-value | ||
| Postprocedural AR | |||||||
| Moderate | 8 (5.4) | 6 (5.5) | 2 (5.3) | 0.999 | 2 (5.9) | 2 (5.7) | 0.999 |
| Severe | 0 | 0 | 0 | 0 | 0 | ||
| Postprocedural mean aortic valve gradient, mmHg | 11 [8-15] | 10 [8-13] | 14 [9-19] | 0.010 | 11 [7-13] | 14 [9-19] | 0.059 |
| Postprocedural peak aortic valve gradient, mmHg | 19 [14-26] | 18 [13-25] | 25 [18-32] | 0.026 | 19 [14-25] | 23 [17-32] | 0.170 |
| Mean gradient >20 mmHg | 12 (8.1) | 5 (4.6) | 7 (18.0) | 0.008 | 1 (2.9) | 6 (17.7) | 0.055 |
| EOA, cm2 | 1.40 [1.20-1.70] | 1.50 [1.30-1.70] | 1.12 [1.00-1.34] | <0.001 | 1.40 [1.29-1.72] | 1.12 [0.97-1.34] | 0.002 |
| Index EOA, cm2/m2 | 0.87 [0.74-1.0] | 0.93 [0.77-1.04] | 0.73 [0.65-0.81] | <0.001 | 0.87 [0.76-1.05] | 0.71 [0.61-0.81] | 0.007 |
| Moderate PPM | 35/125 (28.0) | 22/100 (22.0) | 13/25 (52.0) | 0.003 | 8 (25.0) | 12 (52.2) | 0.039 |
| Severe PPM | 15/125 (12.0) | 9/100 (9.0) | 6/25 (24.0) | 0.039 | 2 (6.3) | 6 (26.1) | 0.057 |
| Any PPM | 50/125 (40.0) | 31/100 (31.0) | 19/25 (76.0) | <0.001 | 10 (31.3) | 18 (78.3) | 0.001 |
| Values are expressed as n (%), n/N (%) or median [IQR]. AR: aortic regurgitation; BEV: balloon-expandable valve; EOA: effective orifice area; IQR: interquartile range; PPM: prosthesis-patient mismatch; SEV: self-expanding valve; THV: transcatheter heart valve | |||||||
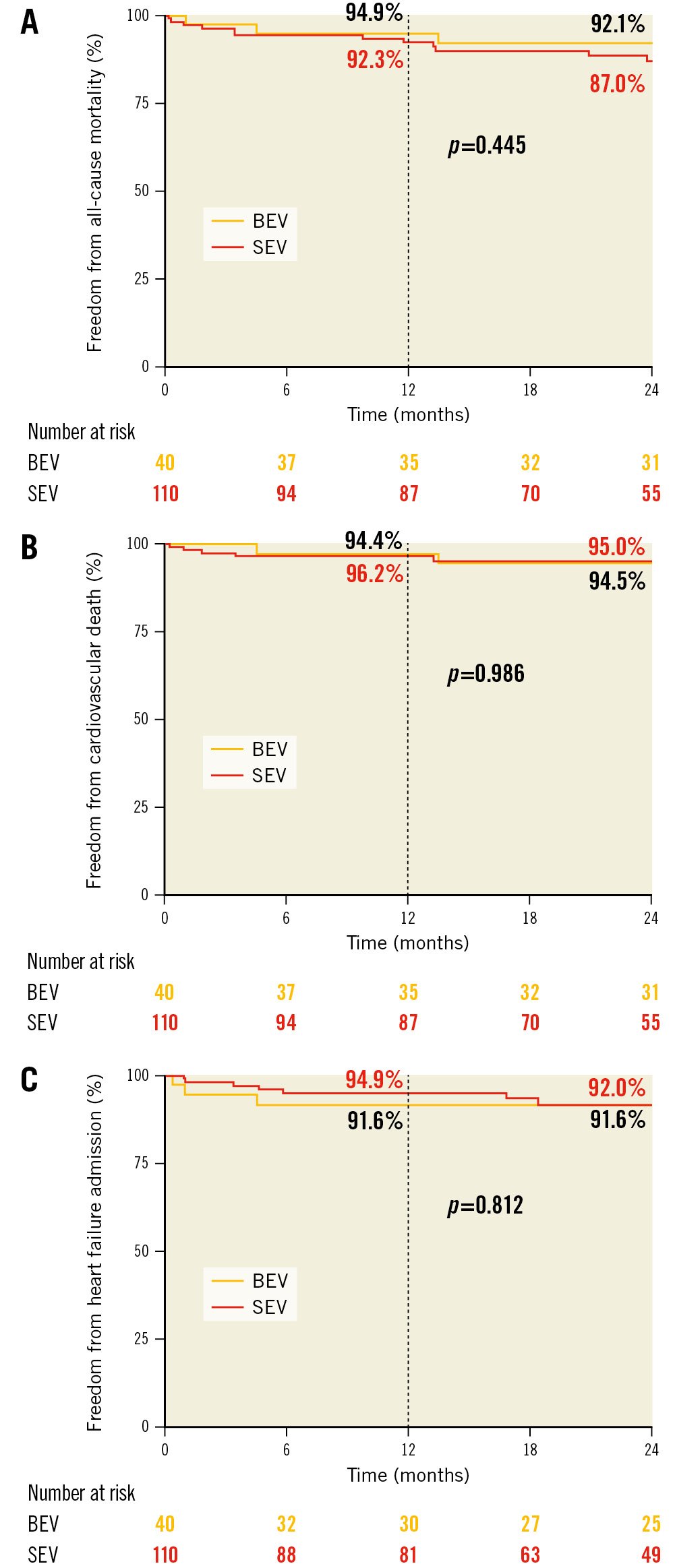
Figure 3. Kaplan-Meier survival curves after transcatheter aortic valve replacement in patients with extra-small aortic annuli. A) All-cause mortality. B) Cardiovascular mortality. C) Heart failure readmission. BEV: balloon-expandable valve; SEV: self-expanding valve
Discussion
TAVI has become the treatment of choice in patients with symptomatic severe aortic stenosis in several clinical scenarios. However, specific anatomical features may increase complexity and limit its use, as in patients with extra-SAA. SAVR is associated with a high risk of severe PPM (37.5%)9 and surgical annulus enlargement is only performed in one-quarter of patients with SAA1819 in experienced surgical centres, and possibly fewer in lower volume centres.
Furthermore, there is no clear consensus on the definition of SAA. In previous TAVI studies, some of the most frequent cutoff points were defined as an area <400 mm2122021, mean diameter <23 mm10222324 or , perimeter <72 mm12 or perimeter <73 mm25. Our study incorporates the definition of extra-SAA (aortic annulus area ≤280 mm2 or perimeter ≤60 mm), which introduces the prospect of a THV to a more challenging clinical scenario. Thus, the use of THV near or below the manufacturers’ threshold in daily practice might represent an off-label indication and might raise concerns regarding the efficacy and safety of TAVI in this setting.
This study analysed the in-hospital and midterm outcomes in a multicentre registry of patients with extra-SAA undergoing TAVI. The main results were that extra-SAA were observed almost exclusively in women (93%), and TAVI was associated with a relatively high rate of technical (>90%) and device (>80%) success at 30 days. Self-expanding systems were more frequently used in this clinical scenario (73.3%) and were associated with higher rates of intraprocedural technical success and 30-day device success, better valve haemodynamics, and similar rates of pacemaker and moderate paravalvular aortic regurgitation. Moderate and severe PPM were more frequent in the BEV group. Despite these differences in periprocedural outcomes, freedom from cardiovascular mortality and from heart failure readmission at 2-year follow-up were not significantly different between the SEV and BEV groups.
Small aortic annuli are predominantly seen in women and are a challenging anatomical feature for both TAVI and SAVR. In TAVI, oversizing the THV could theoretically increase the risk of mechanical complications, valve malposition, or suboptimal valve performance due to THV underexpansion. However, TAVI has shown better haemodynamic outcomes compared to surgery91026, related to the use of a larger prosthesis size, systematic oversizing, and a thinner stent compared to the bulkier sewing ring in stented SAVR. Moreover, previous studies have observed a lower risk of paravalvular leak with TAVI in patients with SAA compared to larger aortic annuli9, possibly attributed to a better sealing mechanism at different levels by the THV. The better haemodynamic results and the low incidence of significant paravalvular leak in SAA have previously been suggested as possible factors for an increased benefit of TAVI versus SAVR in women compared to men5.
To the best of our knowledge, there is only 1 study with a very limited sample size (n=36) that analysed TAVI in extra-SAA (defined as an aortic annulus area <314 mm2), in an Asian population treated with BEV27. They reported no significant increase in periprocedural complications (annular rupture and coronary obstruction of ~1%) with a moderate PPM rate of 22%. In our study the incidence of moderate and severe PPM were higher (28.0% and 12.0%, respectively), which was probably associated with the higher BSA and smaller aortic annulus size in our population. The PARTNER trial reported severe PPM in 19.7% of patients with SAA (annulus diameter <18 mm with transthoracic echocardiography) undergoing TAVI with BEV9. The SAA substudy from the CHOICE-Extend registry (SAA defined as annulus mean diameter ≤23 mm) identified rates of moderate or severe PPM with SAPIEN 3 and Evolut R THVs of 59.2% and 33.3%, respectively22. Similarly, we observed an increased risk of severe PPM with BEV compared with SEV (24.9% vs 9.0%; p=0.039). Balloon post-dilation has been associÂated with a lower risk of PPM28; however, in our cohort, it was performed in only 23% cases, which might be driven by the low rate of paravalvular leak and concern about annulus rupture. Additional observational studies have also observed haemodynamic superiority of SEV compared with BEV in SAA1120212324, which might be confirmed in the ongoing randomised SMART Trial (ClinicalTrials.gov: NCT04722250).
Aortic annulus size and the type of bioprosthesis or THV have been associated with haemodynamic outcomes after SAVR and TAVI5, and severe PPM has been reported as an independent predictor of mortality in patients with SAA and with reduced left ventricular ejection fraction2829, as well as a higher risk of the composite outcome of death, stroke and heart failure admission after TAVI30. Whilst the clinical impact of PPM has not been demonstrated in all of these studies113132, where feasible PPM should be avoided by anticipation of the iEOA, treatment strategy and prosthesis type and size selection33.
Periprocedural complications, including annular rupture, coronary obstruction, or cardiac tamponade are feared complications in patients with SAA. The incidence of coronary obstruction has been previously described as ~1%12232427. In our study, we observed a slightly increased rate of coronary obstruction (2.1%) in patients with a high degree of valve oversizing. Annular rupture occurs in approximately 1% of TAVI34, and it has been associÂated with SAA, aggressive oversizing and BEV34. In our cohort, no cases of overt annulus rupture were observed; however, aortic annulus-contained haematomas occurred in 2 (5.0%) of the patients treated with 23 mm BEV. Of note, the main reason for using 23 mm BEV instead of 20 mm BEV in extra-SAA was the unavailability of the 20 mm device in the participating centres until 2017. Importantly, the overall rate of intraprocedural complications and technical failure was higher in the BEV group compared to the SEV group (22.5% vs 3.6%; p=0.001), suggesting that SEV might be a safer platform in these small anatomies. However, it should be noted that the BEV sample size was relatively small and almost a quarter of the technical failures in the BEV were due to cardiac tamponade in the setting of right ventricular perforation. Right ventricular pacing is used more frequently in BEV implantation, which might increase the risk of right ventricular damage, and impact technical success.
Permanent pacemaker rates were higher compared with large TAVI trials and registries, particularly in the BEV subgroup35. The high degree of oversizing above the recommended range might be one of the reasons for increased conduction disturbances in patients with extra-SAA3637. Finally, moderate paravalvular leak occurred in 5.4%, and cardiovascular mortality and heart failure readmission at 2 years were low (5.2% and 8.0%, respectively). These results are in line with previous studies that included patients with a similar risk profile38. Overall, short- and midterm clinical outcomes from this cohort might support the use of self-expanding valves in TAVI patients with extra-SAA even when these patients have aortic annuli dimensions below the device manufacturer’s threshold. This is particularly relevant when SAVR with annular enlargement is not feasible and/or deemed a high-risk surgical procedure.
Limitations
This study has limitations inherent to observational and retrospective multicentre studies, therefore, differences in device selection and sizing remain. Additionally, the sample size is relatively small, therefore, the power of the study was limited, especially to compare THV performance. A quantitative evaluation of valve calcification (Agatston score) was not available in all patients, and this might have precluded an appropriate assessment of the role of this important factor for valve selection and outcomes. Data regarding baseline conduction disturbances and implantation depth were not available to correct for confounding factors of permanent pacemaker implantation. Although short-term and 2-year outcomes were presented, long-term follow-up is required to further elucidate the impact of SAA on valve durability and PPM on mortality.
Conclusions
In conclusion, patients with severe aortic stenosis and extra-SAA undergoing TAVI were more frequently treated with SEV. This platform seemed to be a safe and effective treatment strategy, with an apparently lower rate of procedural complications, higher device success at 30 days and better haemodynamic valve performance with less risk of severe PPM compared with BEV. However, neither severe PPM nor the type of THV were associÂated with 2-year clinical outcomes.
Impact on daily practice
Small aortic annuli are frequently encountered, especially amongst women considered for aortic valve replacement, increasing the risk of complications and PPM. TAVI, particularly with SEV, has a more optimal haemodynamic performance compared to surgery and should be considered in patients with SAA, especially when SAVR with aortic root enlargement is deemed to be a high-risk procedure. In patients with extra-SAA, near or below the manufacturer-recommended range for THVs, concerns exist regarding safety and efficacy with current THV. Our results suggested a better safety and efficacy profile for SEV compared with BEV, and when possible, this should be considered the first choice in patients with extra-SAA. Further randomised trials are required to confirm these findings and to determine whether improved valve performance and fewer PPM are impactful upon clinical outcomes and durability.
Conflict of interest statement
G. Tirado-Conte holds a research-training contract “Rio Hortega” (CM21/00091) from the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III). O. De Backer received institutional research grants and consulting fees from Abbott and Boston Scientific. L. Asmarats is a proctor for Abbott Vascular and has received speaker fees from Edwards Lifesciences. S. Toggweiler serves as a consultant and/or proctor for Boston Scientific, Edwards Lifesciences, Medtronic, Abbott Vascular, Biosensors, Shockwave, Teleflex, Medira, atHeart Medical, VeoSource, and Polares Medical; has received institutional research grants from Boston Scientific, Biosensors, Fumedica, Medtronic, and Novartis; and holds equity in Hi-D Imaging. D. Arzamendi is a proctor for Edwards Lifesciences and Abbott Vascular. L. Sondergaard is Chief Medical Officer and Divisional VP for Medical Affairs at Abbott Structural Heart; and he has received consultant fees and/or institutional research grants from Abbott, Boston Scientific, Medtronic, and SMT. L. Nombela-Franco is a proctor for Edwards Lifesciences and Abbott Vascular; and holds a research grant (INT19/00040) from the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III). The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

