Transcatheter aortic valve implantation (TAVI) has brought about a paradigm change in the treatment of patients with severe aortic stenosis. There is a growing body of evidence and continuous advancement in the field of transcatheter valve intervention, including expansion to indications other than aortic stenosis, such as pure aortic regurgitation (AR). Currently, the only device approved for transcatheter treatment of pure AR is the Trilogy valve (JenaValve). However, logistical issues and strict anatomical requirements impede its unrestricted use, which is why alternative valve systems must be considered for this subset of patients. Overall, the results of TAVI for pure AR have been worse than for severe aortic stenosis, but there have been improved outcomes with new-generation devices12. The procedural challenges of performing TAVI for pure AR are related to difficult visualisation of the landing zone, the presence of aortic aneurysms, residual AR, and the absence of calcification − required for stable anchoring of the transcatheter heart valve (THV). Hence, the greatest concern for TAVI in pure AR is the increased risk of device embolisation. Registry data demonstrated rates of device malpositioning of 9% versus 33% for early- versus new-generation devices and a high need (16.6% of patients) for implantation of a second valve1.
In this issue of EuroIntervention, Sánchez-Luna et al present a multicentre registry of patients with pure AR undergoing TAVI using the Myval balloon-expandable valve (Meril Life Sciences). A total of 113 cases from 17 international centres were collected. Even though the overall sample size is moderate, this analysis represents the largest of its kind for the indication of pure AR using a balloon-expandable platform3. Furthermore, the work distinguishes itself by the core laboratory analysis of most imaging modalities. Technical success was 95%, residual AR ≥moderate was 3.6% at 30 days, and 30-day all-cause mortality was 5.3%. The procedural success rate was remarkably high despite relatively large annulus dimensions (mean area 638 mm2), as larger annulus dimensions have been associated with lower device success rates1. In a previous multicentre registry, the SAPIEN 3 THV (Edwards Lifesciences) had an 85% device success rate with no case of residual AR ≥moderate and a mean oversizing of 13.6%1. The high success rate in the series reported by Sánchez-Luna et al may be attributed to the greater oversizing (mean cover index 17.0%) with a high proportion of extra-large sizes (Myval 30.5 mm and 32.0 mm) and additional filling volumes of up to 9 ml. The availability of these extra-large sizes allows previously excluded patients to be treated and to optimise oversizing in borderline large cases for conventional sizes. The oversizing did not seem to affect midterm outcomes, but the statement by the authors that there seemed to be a “lack of impact of oversizing on device durability” should be qualified, as no long-term data were available in this study. Furthermore, even though in the present series aggressive oversizing was not at the cost of annular rupture, the limited sample size precludes any conclusions regarding rare complications. Indeed, we have personal experience of a case of annular rupture following aggressive oversizing in a patient with pure AR.
Another highlight of this work is the introduction of a novel classification system of left ventricular outflow tract (LVOT) shape to characterise the risk of embolisation; this is appealing and intuitive. It highlights the importance of a comprehensive understanding of the entire aortic root anatomy and not only the annulus. Hence, tapered LVOT shapes (LVOT size > annulus size) were identified to have a higher risk of embolisation than tubular or flared shapes. This specific knowledge not only may affect preprocedural planning, including valve selection, degree of oversizing, and preparation of appropriate bailout measures, it also mandates a prolonged periprocedural observation, since delayed migration could occur. Notably, for the manufacturers of balloon-expandable devices, the risk of left ventricular migration might be reduced by altering the shape of the THV stent with a flared outflow portion (Figure 1) for improved anchoring by simply modifying the shape of the balloon. This could be easily accomplished but, of course, would require extensive bench and clinical testing.
The data presented do not necessarily apply to other balloon-expandable platforms, especially the Octacor device (Meril Life Sciences) which is the new iteration of the Myval. Nevertheless, the authors demonstrate that TAVI using balloon-expandable devices with sufficient oversizing may represent a viable option for the treatment of inoperable patients with pure AR, with immediate and midterm outcomes that approximate the results of TAVI in severe aortic stenosis.
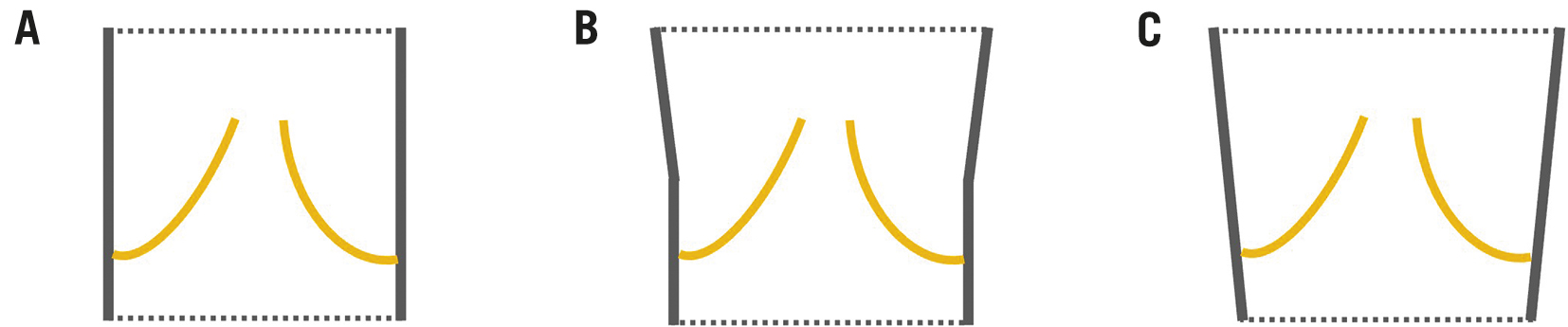
Figure 1. Suggested modification of THV shape. Rather than the standard tubular shape (A), a flared outflow portion as shown in (B) or (C) may improve the anchoring in non-calcified aortic root anatomies.
Acknowledgments
We thank Elizabeth Martinson, PhD, from the KHFI Editorial Office for her editorial assistance.
Conflict of interest statement
W. Kim received personal fees from Boston Scientific, Abbott, Edwards Lifesciences, Meril Life Sciences, and Shockwave Medical; serves on an advisory board at Boston Scientific, Abbott, and HID; and reports institutional fees from Boston Scientific. A. Holzamer received personal fees from Boston Scientific, Edwards Lifesciences, Medtronic, and Meril Life Sciences; serves on an advisory board at Boston Scientific; and reports institutional fees from Boston Scientific.

