Abstract
Aims: We sought to evaluate the outcome of transcatheter aortic valve replacement (TAVR) with the CoreValve Revalving System (CRS-TAVR) in inoperable patients presenting with severe aortic regurgitation (AR), compared to in patients treated for severe native aortic stenosis (AS).
Methods and results: From October 2008 to January 2013, 1,557 consecutive patients undergoing CRS-TAVR, of whom 26 (1.6%) presented with AR, were prospectively followed. Compared with patients with AS, patients with AR were significantly younger (mean age 73±10 vs. 82±6, p=0.02), more frequently in NYHA Class III/IV (95% vs. 73%, p=0.01) and had a higher incidence of severe pulmonary hypertension (sPAP >60 mmHg, 31% vs. 10%, p=0.007). Log EuroSCORE and STS score were similar. VARC-2-defined device success was lower in the AR group (79% vs. 96%, p=0.006). At one month, patients treated for AR had a higher overall mortality (23% vs. 5.9%; OR 4.22 [3.03-8.28], p<0.001) and cardiac mortality (15.3% vs. 4%, OR 4.01 [2.40-7.66], p<0.001). Results were consistent at 12 months: overall mortality (31% vs. 19%, HR 2.1 [1.5-4.41], p<0.001) and cardiac mortality (19.2% vs. 6%, HR 3.1 [2.09-8.22], p<0.001).
Conclusions: CRS-TAVR for AR is associated with a significantly higher mortality compared to CRS-TAVR for AS. Considering the ominous prognosis of these patients when treated medically, TAVR may be a reasonable choice in selected patients. In this regard, conventional risk scores have an inadequate predictive value.
Introduction
Transcatheter aortic valve replacement (TAVR) is an effective option in patients with severe native aortic stenosis (AS) deemed at high or prohibitive risk for conventional surgical aortic replacement1,2.
This technology has not been validated for the treatment of severe aortic regurgitation (AR) and limited data have been reported for severely regurgitant surgical bioprostheses3,4 or native aortic valves5,6. The technological limitations of available devices and reimbursement issues have probably prevented a wider application of TAVR to these patients, despite the known ominous prognosis. Indeed, once symptoms of decompensated heart failure become apparent, mortality without surgical treatment may be as high as 10-20% per year7,8. Surgery is the gold standard treatment for these patients, who are usually much younger compared to the usual TAVR population and with fewer comorbidities. However, in some cases they are deemed inoperable because of the prohibitive risk of mortality/morbidity after surgery and thus considered for TAVR as compassionate therapy.
We therefore sought to evaluate, in a nationwide registry of consecutive patients undergoing TAVR by CoreValve® Revalving System (CRS) (Medtronic, Inc., Minneapolis, MN, USA) implantation, the outcome of inoperable patients admitted with AR without any grade of aortic stenosis, compared to those presenting with AS.
Methods
STUDY DESIGN AND PATIENT POPULATION
From June 2007 until April 2011, a total of 1,557 consecutive patients were treated with the third-generation 18 Fr CRS device in high-volume centres in Italy. Among them, 26 (1.6%) presented with AR, as defined according to current guidelines9, and were treated on a compassionate basis after being deemed to be at a prohibitive surgical risk.
A follow-up plan was scheduled for all patients at one, six, and 12 months, and then yearly. Patients were followed up by means of outpatient clinics and regular contact with general practitioners. Local institutional ethical committees approved this multicentre registry. Eligibility for TAVR was established at each centre based on the consensus of a local multidisciplinary Heart Team which included clinical cardiologists, cardiac surgeons, and cardiac anaesthesiologists. Written informed consent was obtained in all cases.
After comparing patients with AR to those with AS irrespective of the concomitant grade of aortic regurgitation, we then compared the overall and cardiovascular mortality of patients with AR with the subgroup of patients presenting with AS and concomitant moderate/severe AR, as defined by current guidelines9.
DEVICE AND PROCEDURE
The CoreValve prosthesis consists of a trileaflet biological valve sewn into a self-expanding nitinol frame. General anaesthesia, local anaesthesia or mild sedation was decided by the Heart Team during the preoperative meeting. Arterial access (femoral, left or right subclavian), percutaneous puncture or surgical exposure was also determined on the basis of the panel of pre-op imaging tests which in most cases included both angiography and CT scan.
In patients with AR, both CT scan and transoesophageal echocardiography were used to measure the diameter and area of the aortic valve. In the case of discrepancy between CT scan and echo, the measurements according to the CT scan were considered and the prosthesis sized according to the instructions of the manufacturer, i.e., a 10-20% overestimation. The aortic root was investigated with CT scan in all patients.
When treating patients with AR, rapid pacing (150 bpm) was used during the deployment of the CRS.
After the procedure, most patients were managed in an intensive care unit or coronary care unit for at least a day, and a temporary pacemaker (PM) was left in place for at least 48 hours. All patients received acetylsalicylic acid (at least 100 mg before the procedure and lifelong) as well as clopidogrel (300 mg bolus plus 75 mg daily for three to six months unless prolonged administration was required for previous coronary intervention with drug-eluting stents). During the intervention, unfractionated heparin was administered to achieve an activated clotting time of 200 to 250 seconds for the duration of the procedure.
In case of significant concomitant coronary disease, percutaneous intervention was performed before or at the time of TAVR in order to obtain a complete revascularisation.
Endpoint definitions
The primary endpoint of the study was overall mortality at one and 12 months. Cardiac mortality and procedural results were also considered at the same time points. Endpoints were defined according to VARC-2 definitions10. Severe pulmonary hypertension (SPH) was defined as a systolic pulmonary artery pressure >60 mmHg.
Statistical analysis
Continuous variables with normal distribution are presented as mean±standard deviation and compared using a Student’s unpaired t-test for comparisons between groups and a Student’s paired t-test for within-group comparisons. Variables not following a normal distribution were compared with a Mann-Whitney test for comparisons between groups and a Wilcoxon test for within-group comparisons. Categorical variables are presented as counts and percentages, and were compared using chi-squared or Fisher’s exact tests, as appropriate. The cumulative incidences of clinical events at follow-up were assessed with the Kaplan-Meier method and log-rank test.
A Cox regression model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) to compare groups for all-cause mortality, cardiovascular mortality, percentage of NYHA Class III/IV and risk of PM implantation.
All probability values reported are two-sided, and a probability value <0.05 was considered to be significant. All data were processed using the Statistical Package for Social Sciences, version 19 (IBM Corp., Armonk, NY, USA).
Results
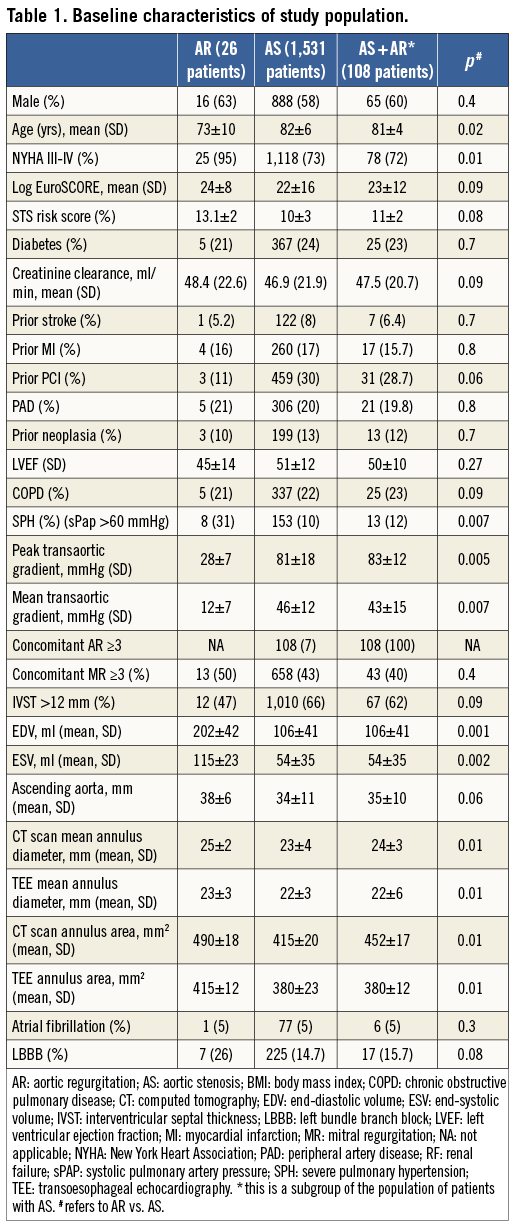
Baseline and procedural features of the study population are shown in Table 1 and Table 2. One-year follow-up was available for the majority of the patients (97%).
Patients undergoing CRS-TAVR for AR were significantly younger, more frequently in NYHA Class III or IV at admission despite medical therapy (95% vs. 73%, and 72% with AS+AR, p=0.01), more frequently had severe pulmonary hypertension (SPH: 31% vs. 10%, and 12% with AS+AR, p=0.007), and, as expected, had larger left ventricle end-systolic as well as end-diastolic volumes. Mean annulus and ascending aorta sizes were also significantly larger in patients with AR (Table 1). The aetiology of AR was: degenerative (19 patients), aortic root aneurysm (five patients), Takayasu’s arteritis (two patients).
The subgroup of patients with AS and concomitant moderate/severe aortic regurgitation consisted of 108 patients. They showed clinical and procedural features quite similar to patients with AS.
Procedural results
In patients treated for AR, the procedure took longer and there was more frequent use of larger CRS sizes (Table 2). We also observed a higher rate of new-onset persistent left bundle branch block in patients with AS compared to AR (14.8% vs. 37.8%, and 36% with AS+AR, p=0.002). On the other hand, the percentage of a second valve implantation, “valve-in-valve”, was higher (19.2% vs. 4.5%, and 4.6% with AS+AR, p<0.001) and subsequently, according to VARC-2 definitions10, that of “device success” was lower (77% vs. 96%, and 96% with AS+AR, p=0.006) in patients treated for AR.
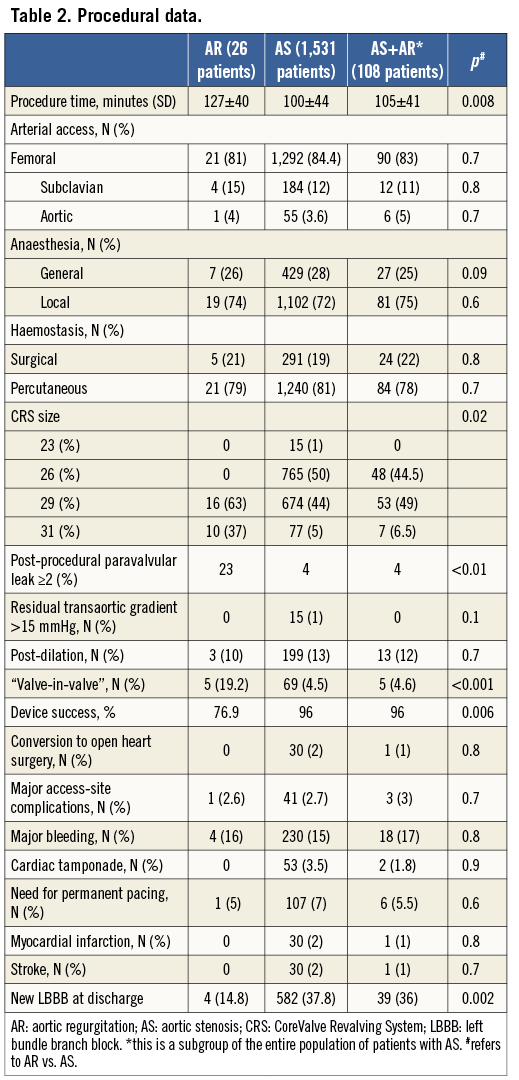
In those five cases (19.2%) in the group of AR in which operators decided to perform a “valve-in-valve” the reasons were: in three cases, despite rapid pacing, the valve shifted towards the outflow tract of the left ventricle; in one case the CRS did not successfully anchor the annulus, thus shifting into the aortic root; and, in one case, the CRS migrated into the outflow tract of the left ventricle.
Of note, in patients treated with AR the rate of paravalvular leak more than mild was significantly higher (Table 2).
Analysis of overall and cardiovascular mortality: AR vs. AS
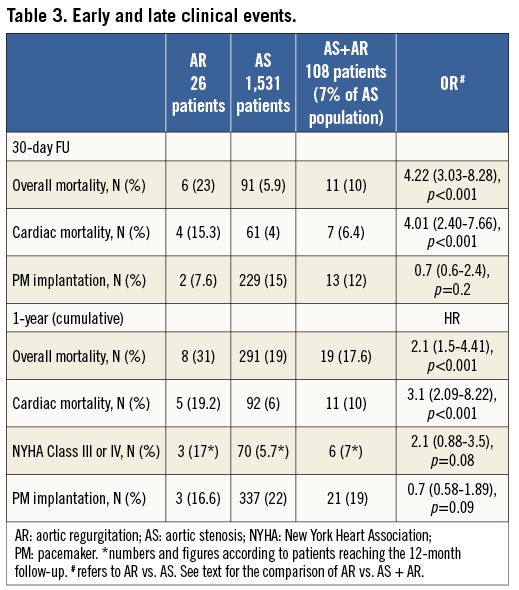
At one month, AR patients had a higher overall (23% vs. 5.9%; OR 4.22 [3.03-8.28], p<0.001]) and cardiac mortality (15.3% vs. 4%, OR: 4.01 [2.40-7.66], p<0.001) (Figure 1, Figure 2).
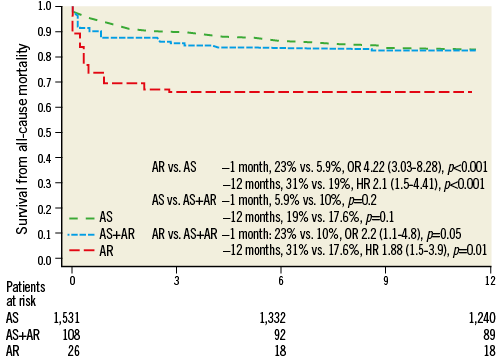
Figure 1. All-cause mortality. AR: severe native aortic regurgitation; AS: severe native aortic stenosis
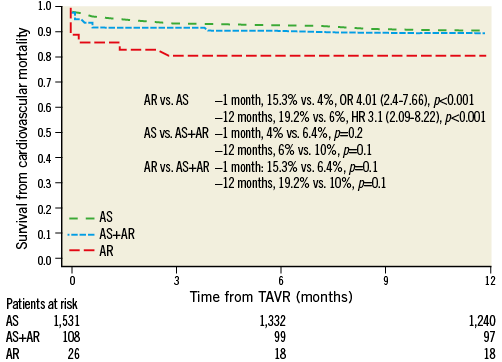
Figure 2. Cardiovascular mortality. AR: severe native aortic regurgitation; AS: severe native aortic stenosis
Likewise, at 12 months patients treated for AR had a higher overall (31% vs. 19%, HR 2.1 [1.5-4.41], p<0.001) and cardiac mortality (19.2% vs. 6%, HR 3.1 [2.09-8.22], p<0.001) (Figure 1, Figure 2).
Analysis of overall and cardiovascular mortality: AR vs. AS plus AR
Patients with AR had a higher risk of overall mortality at one month (23% vs. 10%, OR 2.2 [1.1-4.8], p=0.05) as well as at 12 months (31% vs. 17.6%, HR 1.88 [1.5-3.9], p=0.01) (Figure 1, Figure 2).
Although not reaching statistical significance, patients with AR had a twofold higher risk of cardiovascular mortality at both one (15.3% vs. 6.4%, p=0.1) and 12 months (19.2% vs. 10%, p=0.1) (Figure 1, Figure 2).
PM implantation, NYHA functional class
Although not reaching statistical significance, patients with AR had a twofold lower risk of PM implantation at one month when compared to other groups (Table 3). At 12 months, the percentage of patients in NYHA Class III or IV was higher among those treated for AR but not statistically significant.
Discussion
Patients undergoing CRS-TAVR for AR had a higher overall and cardiac mortality compared to patients treated for AS at both one month and one year. Likewise, with respect to patients with AS plus AR, “pure” AR patients showed a higher risk of both overall and cardiac mortality at one month, but not at one year. The main causes of death in patients treated with CRS-TAVR for AR were arrhythmia, progressive cardiac failure, cardiogenic shock, and pneumonia.
On the other hand, patients with AR showed a lower rate of PM implantation but a higher, although not statistically significant, rate of NYHA Class >III at one year.
From a procedural point of view, CRS-TAVR proved to be more challenging as reflected by the higher rate of “valve-in-valve” and the lower rate of procedural success.
The implementation of TAVR has resulted in a paradigm shift in the treatment of patients with severe aortic stenosis deemed at high or prohibitive risk for surgery1,2.
Of note, a “pure” severe aortic stenosis is rather exceptional, as the majority of the patients show a variable grade of aortic regurgitation along with a severe transvalvular gradient11-14.
TAVR is strictly indicated for the treatment of severe aortic stenosis; nevertheless, such a coexistence of aortic regurgitation did not hamper the results achieved with both available transcatheter bioprostheses1,2,11-14.
TAVR for AR is still “off-label” and only limited experiences are available in the literature5,6. Our multicentre registry shows that inoperable patients with AR have a significantly worse prognosis compared to a contemporary consecutive cohort of patients with AS, with or without a concomitant moderate/severe AR. Of note, common risk scores, such as the STS and logistic EuroSCORE, did not differ significantly across the groups, meaning that their performance in predicting mortality/morbidity is inadequate in this particular setting.
AS is a consequence of the calcific degeneration of the valve apparatus leading to hypomobility of the leaflets, which may even merge, thus generating the conditions for a high transvalvular gradient. This mechanism is also responsible for the coexisting variable grade of regurgitation.
The pathophysiology of AR is different. Indeed, possible causes of AR range from congenital disease to infective causes, rheumatoid vasculitis, Takayasu’s arteritis, radiotherapy, chronic dissection and so on.
Furthermore, patients with AR are anatomically different. Indeed, the echocardiographic parameters showed a significantly larger aortic annulus, end-diastolic volume and end-systolic volume and, as expected, significantly lower transaortic gradients.
Compared to patients with AS, patients with AR are also clinically different. They are younger, more frequently in a higher NYHA class, more frequently have an SPH (>60 mmHg) and, most importantly, they are all truly inoperable.
Given these premises which depict a different physiopathological, anatomical, and clinical scenario with respect to TAVR for AS, the observed results need to be evaluated with great caution.
The current treatment of choice for patients with AR remains surgical aortic valve replacement.
In this study, the transcatheter therapy showed a lower rate of procedural and device success than that observed in patients with AS, presumably as a consequence of the anatomical features of these patients, the lack of severe calcifications determining an inefficient anchoring of the CRS, and a high volume regurgitant jet causing gross movements of the prosthesis during the release.
Notwithstanding the consistent use of rapid pacing during deployment across the study centres, these anatomical features may actually explain the higher rate of “valve-in-valve” as compared to the AS group, despite the fact that these procedures were performed by the same expert operators.
The 31 mm CRS was introduced after the start of the registry. It actually allowed the treatment of patients with larger annuli without any learning-curve effect.
The lower but not statistically significant rate of PM implantation and the lower rate of new-onset LBBB may also have an anatomical explanation. Indeed, as patients with AR presented with larger annuli and left ventricle sizes, it can be presumed that, on the basis of the “compression/inflammation hypothesis”, they are less prone to suffer an impairment of atrioventricular conduction15.
In AR cases, operators more often opted for a “valve-in-valve” implantation. Moreover, in these patients we observed a significant paravalvular leak in a much higher percentage despite the fact that all the operators adhered strictly to the manufacturer’s indication of a 10%-20% CRS annulus oversizing, and the aortic valve as well as the aortic root were evaluated with both CT scan and transoesophageal echocardiography.
Besides some preliminary reports16-18, literature in this field consists of only one recent registry6. In the latter, Roy et al collected cases performed in different countries and without a head-to-head comparison with contemporary patients presenting with AS. The rates of mortality they reported (9.3% and 21.4%, at one month and one year, respectively) are lower than those we observed in our unselected population. A possible explanation may be related to the lower risk of mortality according to the log EuroSCORE and the STS score of the population analysed by Roy et al, though these scores have an insufficient predictive performance in TAVR patients. Indeed, they reported a log EuroSCORE of 26.9±17.9 and an STS score of 10.2±5.3, while we reported 24±8 and 13.1±2, respectively. The amplitude of the standard deviations is obviously a consequence of the very low score of some patients included by Roy et al.
Of note, the present study did not select specific cases; however, it is the first nationwide registry directly comparing the outcome of consecutive patients undergoing CRS-TAVR for AR compared to a contemporary cohort of patients with AS.
Avenues for future technology
Although showing the feasibility of the procedure, our data also highlighted some technical issues. When selecting the prosthesis size, the indication for a 10-20% oversizing with respect to the aortic annulus may not be enough, considering that this subset of patients often shows a large LV outflow tract and large aortic roots. However, only specifically designed studies may provide insights into this issue.
The high rate of cardiac mortality deserves consideration: it appears that these patients are treated when already at an end stage of the disease, i.e., at a stage where the clinical equipoise of the TAVR procedure could even be questionable. As a consequence, in order to obtain better results, more efforts should be devoted to following these patients on the clinical ambit, before they reach a stage of the disease when the cardiac mortality is too high to be improved by the TAVR procedure. From a technical point of view, given that a real alternative to surgery is still lacking for inoperable patients with AR, certain device improvements and advancements need to occur in order to provide these patients with a transcatheter treatment that could be truly beneficial. In particular, future research should focus on fully retrievable devices (thus minimising the rate of malpositioning and subsequent need for “valve-in-valve”), which should also be able to fit into the aortic annulus better, even in the absence of evident calcifications.
Limitations of the present study
This was a very large multicentre registry. There was no randomisation; however, no selection bias was likely as every patient was added to this nationwide registry. Although there was no central adjudication of events, the Valve Academic Research Consortium-2 definitions were adopted to standardise results. Moreover, from the beginning this database has included the first TAVR centres in Italy with a high level of expertise and case volume.
The small number of patients with AR does not allow definite conclusions, and the statistical significance of some results may have been conditioned by the diversity of the two groups. However, in contrast to previous reports, the present comparison with a contemporary cohort of patients treated “on-label” appears to be necessary to depict better the scenario in which these procedures have been performed.
The present data relate to a large cohort of patients treated with CRS, and no extrapolation can be made with respect to the procedural performance of the Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA) or even newer generations of transcatheter bioprostheses.
Conclusions
At present, surgery is still the gold standard treatment for patients with AR. However, considering the ominous prognosis of this condition when treated conservatively, TAVR may have a role in highly selected inoperable patients. There are several technical issues with the current generation of CRS, and there is still insufficient experience to achieve results similar to those obtained in the context of severe aortic stenosis.
|
Impact on daily practice The gold standard treatment for patients with severe aortic regurgitation is the surgical approach. However, the present study shows that TAVR may have a beneficial role, considering the ominous prognosis of inoperable patients. |
Conflict of interest statement
F. Bedogni, G. Ussia, and A. Petronio are TAVR medical proctors for Medtronic. A. Latib is a consultant for Medtronic and Direct Flow Medical. However, Medtronic had no role in study design and analysis, decision to publish, or preparation of the manuscript. The other authors have no conflicts of interest to declare.

