Abstract
Aims: The aim of this study was to provide a real-world snapshot of contemporary Heart Team decision making on patients with aortic stenosis (AS) and the consequent short-term clinical outcome.
Methods and results: This was an international multicentre prospective registry encompassing 390 patients with symptomatic severe AS who were prospectively enrolled. Clinical endpoints and the decisive arguments to opt for surgical or transcatheter aortic valve replacement, or medical therapy were recorded separately. The mean age was 76.4±11.6 years, 55% were male and the STS score was 2.9% (IQR 1.6-6.9). The local Heart Teams considered 43%, 25% and 23% to be at low, intermediate and high operative risk with a calculated STS score of 2.18±1.72, 5.08±2.76 and 13.15±9.43, respectively. Overall, 7% were deemed inoperable. Ninety-four percent of patients at low operative risk were sent for SAVR whereas 64% and 92% of intermediate and high-risk patients underwent TAVI. Only 6% of patients did not receive any kind of aortic valve replacement. Overall, 30-day all-cause mortality was 2.8%. TAVI was associated with more major vascular complications, need for permanent pacemakers and post-procedural aortic regurgitation. SAVR had more life-threatening bleedings and new-onset atrial fibrillation.
Conclusions: The PRAGMATIC AS survey offers a snapshot of the contemporary management of patients with symptomatic severe AS. Multidisciplinary Heart Teams select an optimal strategy based on age, frailty and comorbidities. Nearly half of all patients are sent for TAVI. Only a small minority of patients will not receive valve replacement therapy.
Introduction
Symptomatic severe aortic stenosis (AS) is the most common valvular heart disease requiring valve replacement in the adult population of Western societies. Apart from symptomatic relief, aortic valve replacement improves long-term survival1,2.
Prior to the introduction and adoption of transcatheter aortic valve implantation (TAVI), up to 40% of patients with severe symptomatic AS did not undergo aortic valve replacement because of old age, LV dysfunction or comorbidities3.
TAVI is a less invasive alternative to surgical aortic valve replacement (SAVR) and may be considered in patients with a (very) high estimated risk of mortality or complications after SAVR4,5. The explosive uptake of TAVI has evoked a paradigm shift in the treatment of patients with degenerative AS. Updated joint guidelines on valvular heart disease from both sides of the Atlantic formulate several recommendations related to TAVI: 1) a decision for TAVI requires a multidisciplinary Heart Team discussion and consensus; 2) TAVI should be considered for inoperable patients with AS and a life expectancy exceeding one year (class IB recommendation) or can be an alternative to SAVR in patients with a high operative risk (class IIA recommendation)4,5. Established surgical risk models, such as the Society of Thoracic Surgeons (STS) score and logistic EuroSCORE perform relatively poorly in the (very) high-risk populations currently considered for TAVI6. Furthermore, novel insights into these particular patient populations have revealed relatively common risk variables that are not considered in these models yet influence the Heart Team consensus7,8. The aim of the Pooled-RotterdAm-Milano-Toulouse In Collaboration Aortic Stenosis (PRAGMATIC AS) survey was to provide a real-world snapshot of contemporary Heart Team decision making and the consequent clinical implications and outcome in patients with severe degenerative AS.
Methods
All consecutive patients above 50 years old with symptomatic severe degenerative AS who presented to the multidisciplinary Heart Teams of the Erasmus Medical Center in Rotterdam, Clinique Pasteur in Toulouse, Hôpital Rangueil in Toulouse and San Raffaele Scientific Institute in Milan from 1 February to 30 April 2014 were enrolled in a prospective web-based electronic database. The multidisciplinary Heart Teams consisted of a core minimum of a cardiac surgeon and an interventional cardiologist but in general were completed by imaging specialists, anaesthesiologists and other specialists involved. Severe AS was defined by an aortic valve area (AVA) ≤1.0 cm2 (or aortic valve area index [AVAi] ≤0.6 cm2/m2) with a mean transvalvular gradient >40 mmHg (or Vmax >4.0 m/sec by resting echocardiogram [or dobutamine stress echocardiogram, if the subject had a left ventricular ejection fraction (LVEF) <55%]) or time velocity ratio <0.25. Congenital AS (except bicuspid aortic valve disease), any other concomitant severe valve disorder requiring surgical valve treatment and moderate AS for which SAVR was planned in combination with other cardiac surgery (coronary artery bypass grafting [CABG], valve replacement, ascending aorta) were formal exclusion criteria.
Patient demographics, estimated surgical risk by calculating STS, logistic EuroSCORE and EuroSCORE II and other variables which were considered by the respective local Heart Teams were collected in electronic case report forms (eCRF). We also captured how frailty was assessed. Eye-ball testing, Katz index, walking-aid dependence, gait speed, hand-grip testing were recorded if applied. The decisive arguments to opt for SAVR, TAVI or medical therapy were recorded separately. All-cause mortality and disabling stroke at 30 days were also collected with time 0 being the procedure day (for SAVR and TAVI) or the day of final decision to defer any invasive procedure by Heart Team consensus. Mortality and stroke data after hospital discharge were collected from hospital charts and by contacting the respective national civil registry, referring physician or general practitioner. Other endpoints were collected according to the most recent Valve Academic Research Consortium (VARC) endpoint definitions9. In accordance with the Declaration of Helsinki, the respective institutional review boards approved the prospective data collection. All patients who underwent surgical or catheter-based treatment provided written informed consent for the procedure.
STATISTICAL ANALYSIS
Categorical variables are presented as frequencies and percentages, and compared with the use of the Pearson’s chi-square test or the Fisher’s exact test, as appropriate. Continuous variables are presented as means (±SD) in case of a normal distribution, or medians (IQR) in case of a skewed distribution, and compared with the use of analysis of variance. Normality of the distributions was assessed using the Shapiro-Wilk test. A two-sided alpha level of 0.05 was used for all superiority testing. The statistical analyses were performed using SPSS software, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 467 patients were enrolled (Figure 1). Thirty-four patients did not meet the inclusion criteria and were therefore removed from further analysis, leaving a total of 433 patients. Due to loss to follow-up and missing data, 43 patients were not included, leading to the final population of 390 patients. The relative proportion of patients with incomplete data was similar for the three treatment cohorts (OMT 17%, TAVR 14% and SAVR 18%, p=0.47). The division among the participating centres was as follows: Clinique Pasteur n=145 (37%), Hôpital Rangueil n=109 (28%), Erasmus Medical Center n=68 (17.5%), and San Raffaele Scientific Institute n=68 (17.5%). Overall, 51% of patients underwent SAVR, 43% TAVI and 6% medical therapy (Figure 2). Relative proportions among therapies (SAVR: TAVI: medical therapy) varied across the centres: Pasteur 55:40:5; Rangueil 63:32:5, Erasmus 40:38:12 and San Raffaele 35:59:6, respectively.
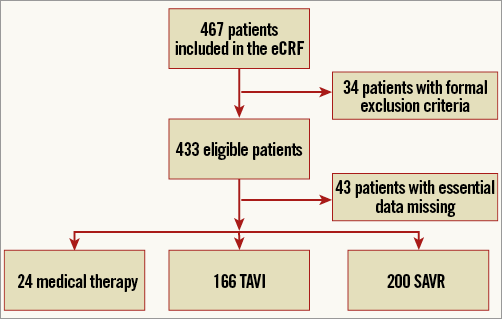
Figure 1. Patient flow chart. SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation
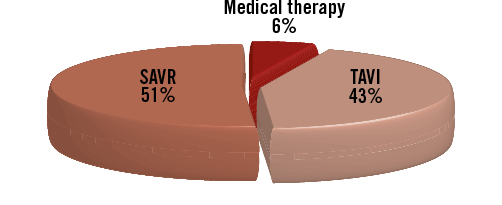
Figure 2. Relative proportion of surgical aortic valve replacement (SAVR), transcatheter aortic valve implantation (TAVI) and medical therapy.
Table 1 displays the baseline demographics including substratification according to the selected treatment strategy.
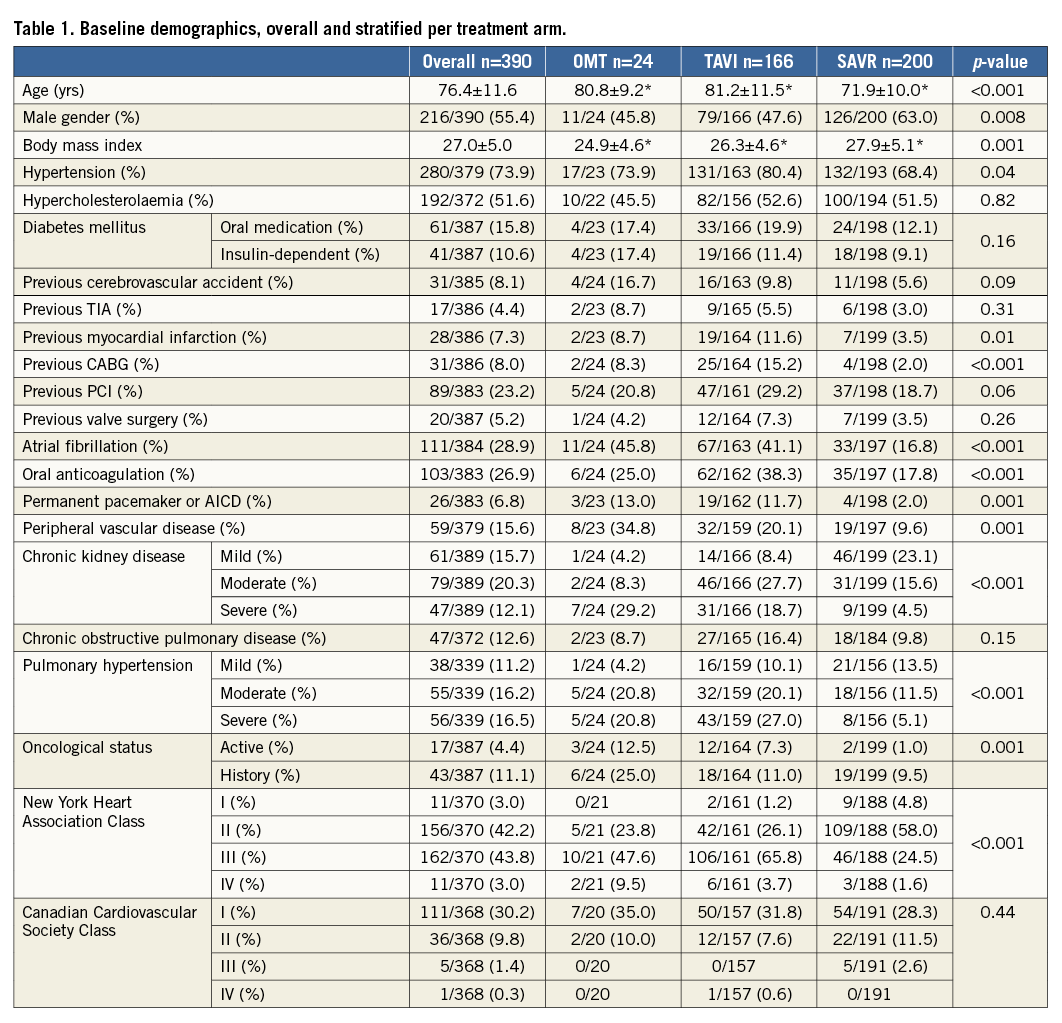
Mean age was 76.4±11.6 years, 55% were male and mean STS PROM score was 2.9 (1.6-6.9). Patients selected for SAVR were more often female (63% vs. 54% for medical therapy and 52% for TAVI, p=0.006), younger (71.9±10 vs. 80.8±9.2 for medical therapy and 81.2±11.5 for TAVI, p<0.001) and had less cardiovascular antecedents or moderate/severe chronic kidney disease. Conversely, patients selected for TAVI or medical therapy had more pulmonary hypertension, AF, permanent pacemakers and underlying active malignancy. Poor LV function and more than moderate aortic or mitral regurgitation were more frequent in patients with medical therapy (Table 2).
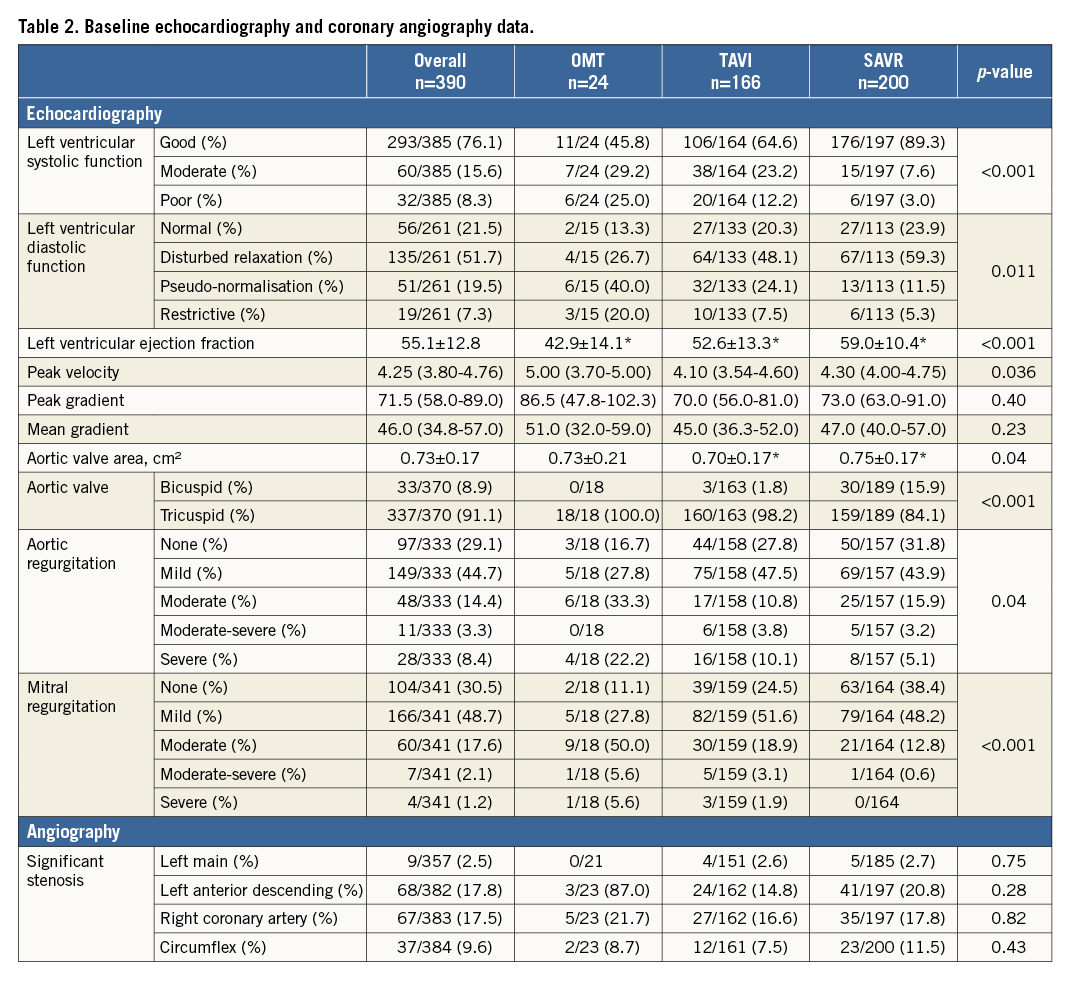
Significantly, more patients undergoing SAVR had a bicuspid aortic valve. SAVR patients were more often in NYHA Class II as compared to NYHA Class III or Class IV in patients selected for TAVI or medical therapy. Patient risk estimation is detailed in Table 3.
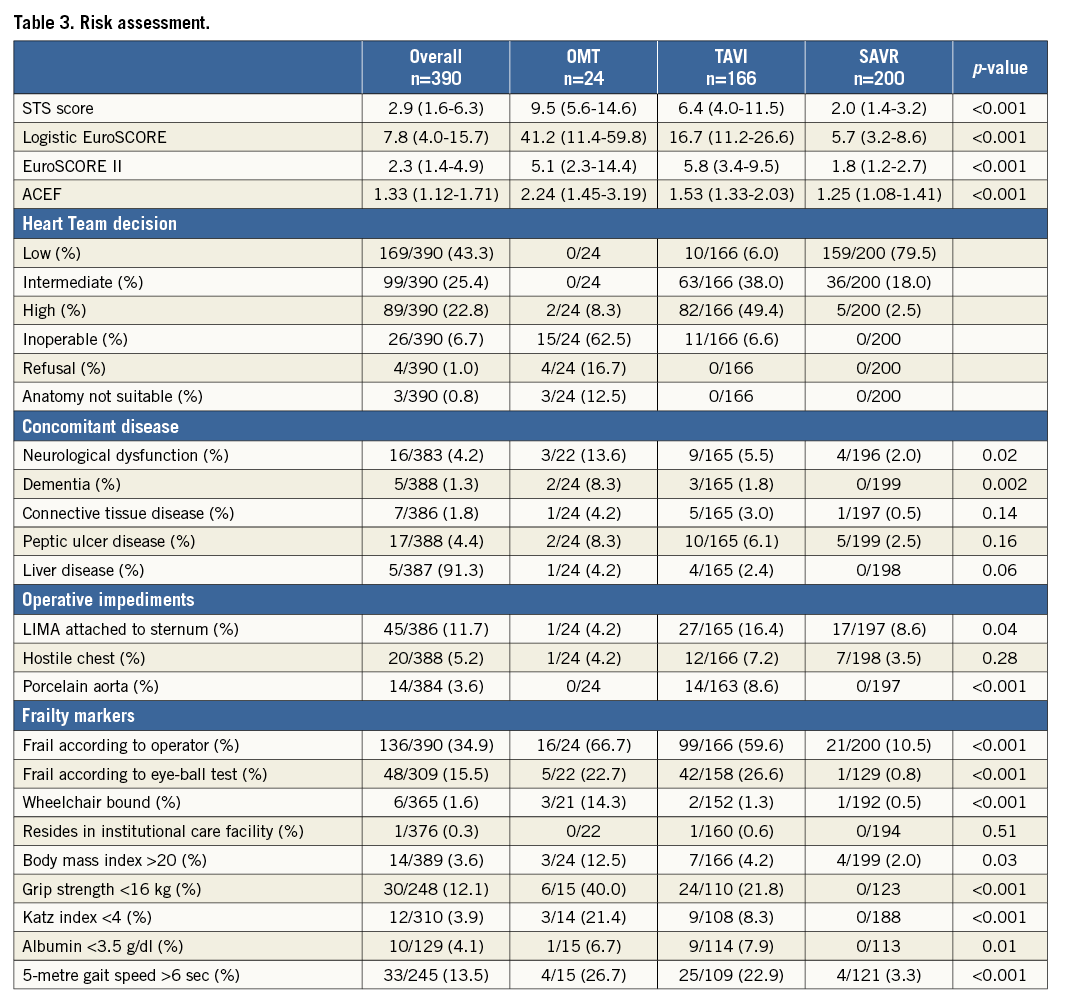
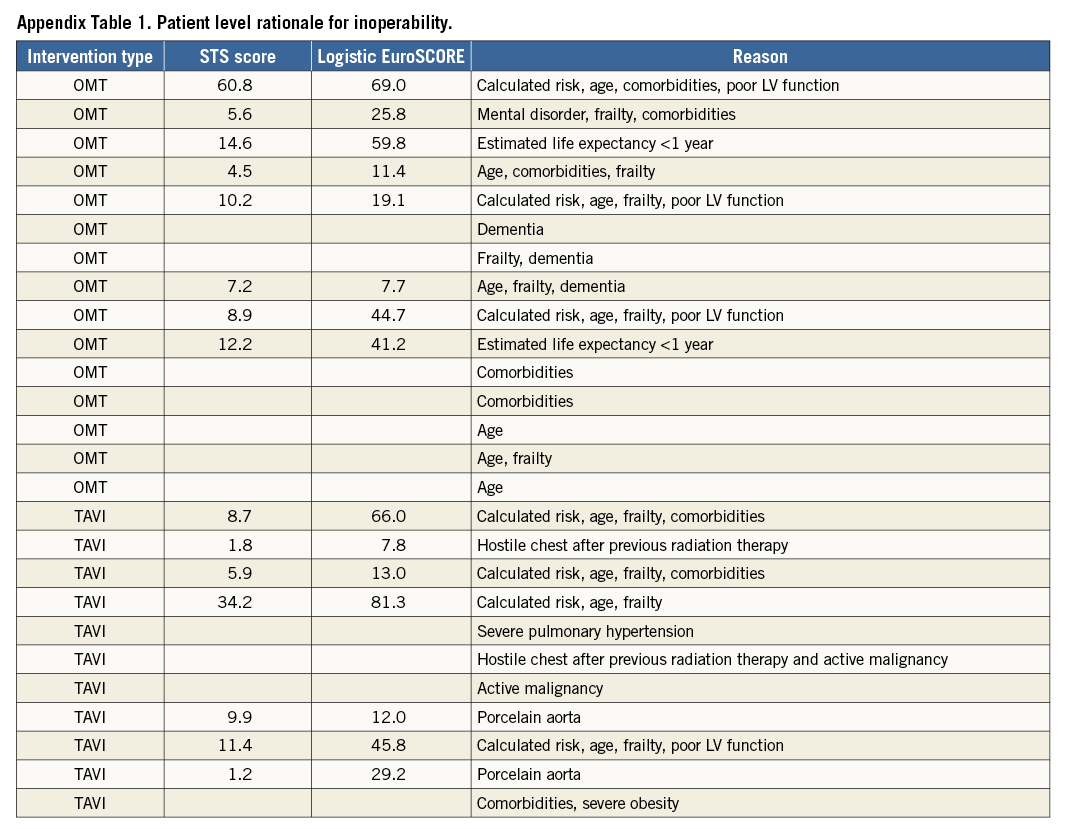
The local Heart Teams considered 43%, 25% and 23% at low, intermediate and high operative risk with a calculated STS score of 2.18±1.72, 5.08±2.76 and 13.15±9.43, respectively. Overall, 7% were deemed inoperable. The rationale to deem a patient inoperable is described in Appendix Table 1. Calculated surgical risk was significantly higher with medical therapy and TAVI with STS PROM 9.5% (5.6-14.6) and 6.4% (4.0-11.5) vs. 2.0% (1.4-3.2) for SAVR patients (p<0.001), respectively. Almost all patients at low operative risk (94%) underwent SAVR and nearly 80% of patients undergoing SAVR were at low risk. Of the 99 patients at intermediate risk, 64% underwent TAVI and 36% SAVR. The majority of patients at high risk were sent for TAVI. Overall, 26 patients (7%) were considered inoperable and treated medically in 58% and with TAVI in the remainder. Operative risk was more than intermediate in 56% of the TAVI cohort. Table 3 details the reasons for inoperability. Overall, 35% of patients were considered frail with higher relative proportions in patients with medical therapy (67%) and TAVI (59%). Frailty was predominantly a clinical diagnosis rather than based on physical testing or frailty risk calculation. Anatomical impediments for SAVR were relatively scarce. All patients with porcelain aorta (8%) underwent TAVI. Of note, LIMA attachment to the sternum was never considered a formal reason for inoperability.
Procedure
Procedural details are displayed in Table 4 and Table 5. Of the SAVR patients, 8% received a mechanical prosthesis, 73.5% a stented bioprosthesis and 16.5% a stentless bioprosthesis. Coronary revascularisation was more frequent in patients undergoing SAVR (34% vs. 10.8%).
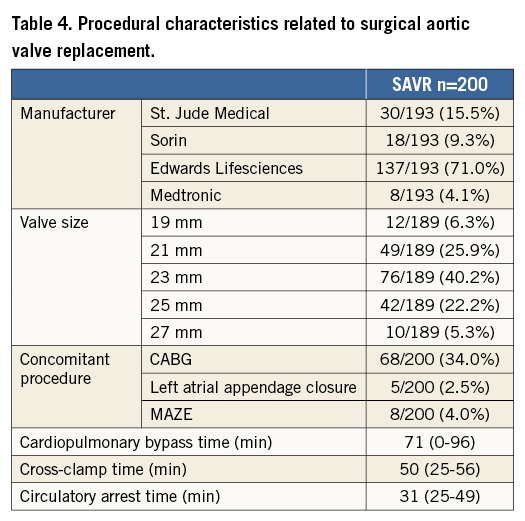
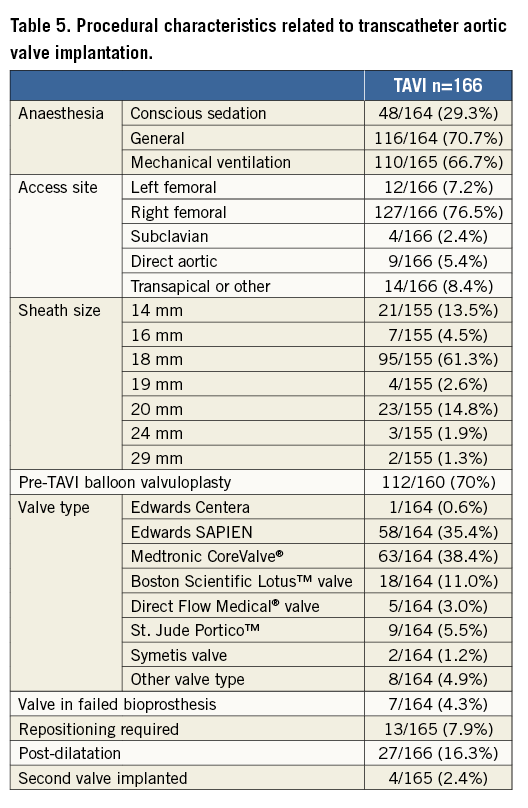
TAVI evolved under general anaesthesia in 71% of cases using primarily a transfemoral approach (83%). Balloon predilatation was performed in 71% of cases. The Edwards balloon-expandable valve (Edwards Lifesciences, Irvine, CA, USA) and the self-expanding CoreValve® (Medtronic, Minneapolis, MN, USA) were most frequently used. Balloon post-dilatation was needed in 16% of cases.
Clinical outcome
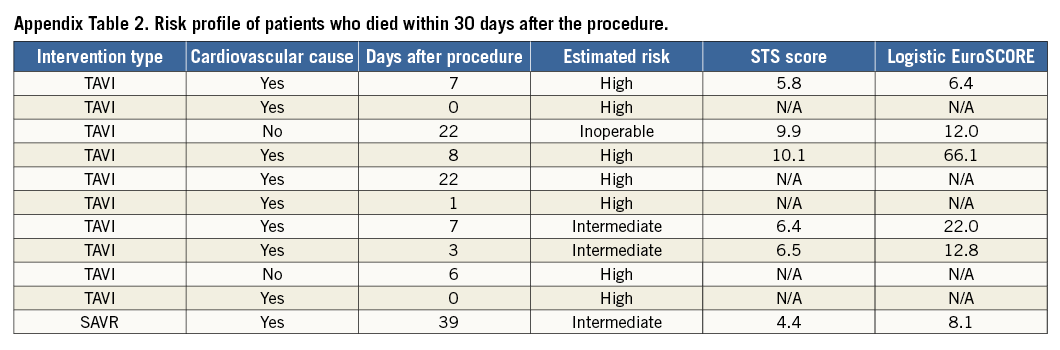
Overall, 30-day all-cause mortality was 2.8% (TAVI 6% vs. SAVR 0.5%) (Table 6).
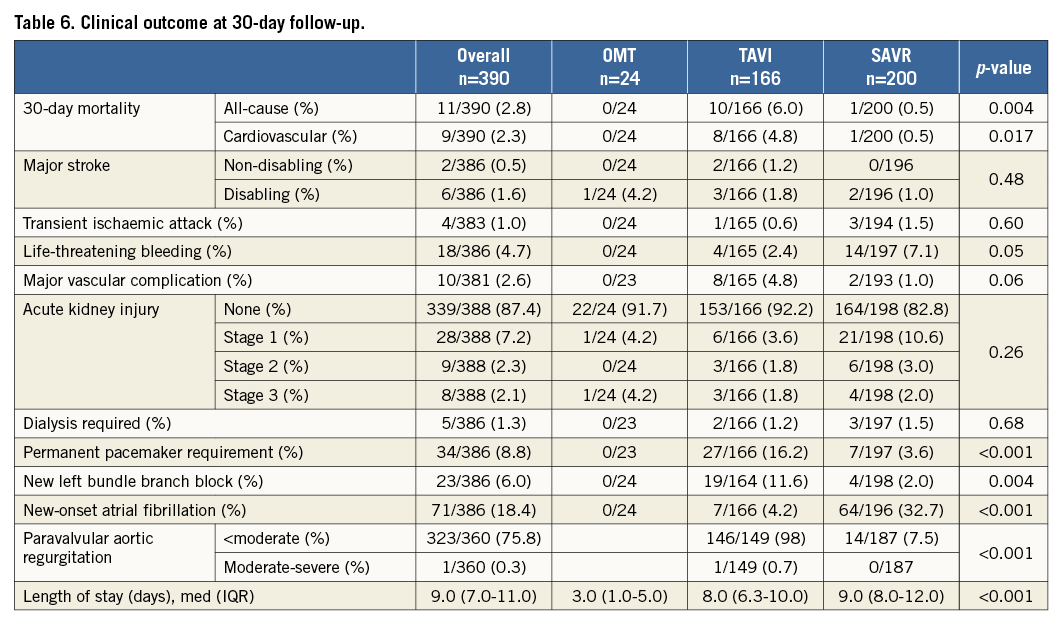
The background risk profile of the patients who died is displayed in Appendix Table 2. TAVI was associated with significantly more major vascular complications, need for permanent pacemakers and post-procedural aortic regurgitation. SAVR had more life-threatening bleedings and new-onset atrial fibrillation. Length of hospital stay with SAVR was nine days (IQR 8-12) vs. eight days (IQR 6.3-110) with TAVI (p<0.001).
Discussion
The prospective PRAGMATIC AS survey represents a snapshot of contemporary management of patients with degenerative AS in selected countries in Europe and highlights the following. 1) Risk estimation was primarily based on Heart Team decision. 2) Overall, nearly half of the patients were considered at low operative risk (these patients were predominantly sent for SAVR), a quarter of patients were at intermediate risk (two thirds of these underwent TAVI), and almost all patients at high risk were sent for TAVI. 3) Overall, 30-day all-cause mortality was low, being highest for patients at higher operative risk, and neurological events were rare. 4) TAVI was associated with more permanent pacemaker implantations and shorter hospital stay compared to SAVR.
The average STS score of 2.9% and the mean age of 76 years in the PRAGMATIC AS survey are evidently higher than those described in other comprehensive AS registries because we deliberately excluded patients below 50 years of age. A recent analysis from the STS database on 141,905 patients undergoing SAVR mainly for AS presented a mean age of 67.6 years and a median STS score of 1.8%10. Furthermore, the Euro Heart Survey studied patients with severe native valvular heart disease and a mean age of 65±14 years with 16.8% <50 years1. In the PRAGMATIC AS survey, 43% of patients were deemed low operative risk and 25%, 23% and 7% intermediate, high operative risk and inoperable, respectively. This relative proportion of low-risk patients differs from the previously mentioned STS database study which judged 80% of patients at low risk, defined by an STS score <4% with a mean STS score of 1.67±0.94. The exclusion of patients below 50 years and a changing risk scoring paradigm may explain this difference. Indeed, in the PRAGMATIC AS survey the individual patient’s operative risk was determined by the multidisciplinary Heart Team rather than by relying on surgical risk models4,5. Established surgical risk calculators perform poorly in elderly patients with multiple comorbidities6,11-14. However, surgical risk models are still valuable in characterising larger study cohorts and, rather than being decisive, should complement the Heart Team discussion process8.
Overall, half of the patients underwent SAVR, 43% TAVI and 6% medical therapy. In the German Aortic Valve Registry (GARY) encompassing 13,860 AS patients from 78 sites and covering 55% of all aortic valve interventions nationwide in the year 2011, 72% underwent SAVR and 28% TAVI15. Similar to the PRAGMATIC AS survey, SAVR patients were largely 10 years younger with fewer comorbidities. The PRAGMATIC AS survey suggests that TAVI filled a void that existed in clinical AS practice, as only 6% of patients were treated medically in contrast with one third of the patients in the Euro Heart Survey more than a decade ago1,3.
In the PRAGMATIC AS survey, two thirds of patients treated medically or with TAVI were considered frail based on clinical judgement. Importantly, frailty indices were not frequently applied in real practice. Patients were inoperable predominantly due to a combination of frailty and multiple comorbidities. A recent analysis of the inoperable arm (cohort B) of the PARTNER (Placement of Aortic Transcatheter Valve) trial revealed that one quarter of patients were deemed technically inoperable and the others clinically inoperable due to frailty or multiple comorbidities16. Also, in the US CoreValve extreme and high-risk study, frailty was common, illustrated by a 5-metre gait speed >6 seconds in 80% of patients17,18. Furthermore, in the comprehensive Society of Thoracic Surgeons/American College of Cardiology (STS/ACC) Transcatheter Valve Therapies Registry on 12,182 patients from 299 US hospitals with a median STS score of 7.1%, 40% were considered frail with a 5-metre gait speed >6 sec19.
In the PRAGMATIC AS survey, TAVI was the preferred strategy in almost all patients deemed at high risk and in two thirds of intermediate-risk patients. Faster ambulation, shorter recovery time, quicker improvements in quality of life and patient preference may stimulate growing adoption of TAVI and a shift towards a more liberal use of TAVI. Data from the Munich Heart Center from 2010 already suggested a trend towards treating lower-risk patients (mean STS score of 4.8±2.6%) with TAVI20. In addition, Wenaweser et al reported on a TAVI cohort of 389 consecutive patients. Almost half of this cohort had an intermediate surgical risk per Heart Team decision with an STS <3% in 10% and STS ≥3% and ≤8% in 65%21. The Bern-Rotterdam-Munich (BERMUDA) study compared 255 propensity-matched pairs of patients undergoing either TAVI or SAVR with an intermediate risk defined as an STS score between 3% and 8%. There was no difference in all-cause 30-day (7.8% vs. 7.1%, p=0.074) and one-year mortality (16.5% vs. 16.9%, p=0.64)22. Patient selection for TAVI seems to be driven by age rather than by the calculated operative risk since in both the GARY registry and the PRAGMATIC AS survey the average age of the TAVI cohort was 10 years higher as compared to the SAVR cohort (81.2±11.5 vs. 71.9±10).
Transfemoral access with either a balloon-expandable or self-expanding THV was the predominant TAVI strategy. In 2014 other transfemoral THV designs became available and will undoubtedly acquire market share in the near future. The majority of TAVI cases evolved under general anaesthesia. In the 2011-2012 pilot European Sentinel Registry of TAVI, encompassing 4,571 patients from 137 centres in 10 European countries, general anaesthesia was used in 63% of cases as compared to 44% in the GARY registry15,23.
The all-cause mortality rate at 30 days in the entire PRAGMATIC AS survey was 2.8% and is similar to the 3% in the STS database10. The SAVR cohort with an STS PROM score of 2% showed a 30-day mortality rate of only 0.5%, which compares favourably with the 1.9% 30-day mortality rate in the low-risk STS database subset with an STS PROM score of 1.46%. The TAVI cohort that covered the entire spectrum of operative risk had an estimated STS PROM score of 6.4% and an actual 6% 30-day mortality rate. In the ADVANCE study, 1,015 patients with a median STS PROM score of 5.1% (IQR 3.6-7.8) and age 81.1 years, the 30-day all-cause mortality rate was 4.5%. In the STS/ACC Transcatheter Valve Therapies Registry, the 30-day mortality rate was 7% (CI: 6.5%-7.4%) with a median STS score of 7.1%. The GARY registry reported in-hospital mortality of 2.1% to 4.5% after SAVR and 5.1% to 7.7% after TAVI15,24. Wenaweser et al reported gradually higher all-cause mortality per STS score tertiles: 2.4% in the cohort with STS score <3%, 3.9% in the cohort with STS score 3%-8% and 14.9% in the cohort with STS >8%21. The PRAGMATIC AS survey corroborates findings from randomised trials comparing TAVI and SAVR in high-risk patients17,25: in comparison with SAVR, TAVI was associated with more vascular complications and need for permanent pacemaker but with less life-threatening bleedings and new-onset atrial fibrillation. Neurological events were relatively scarce in both treatment arms. Furthermore, length of stay appeared shorter after TAVI.
Limitations
The PRAGMATIC AS survey only considered patients who were referred to the respective departments of cardiology and cardiothoracic surgery. Therefore, this survey is susceptible to referring practices. This may, for instance, result in underrepresentation of inoperable patients who were not referred for treatment. The participating centres prospectively entered all data in an eCRF; however, there was no independent clinical events committee. Data follow-up was complete for 90% of patients. The remaining 10% was equally distributed over the different treatment modalities (OMT 17%, TAVR 14% and SAVR 18%, p=0.47). We therefore believe our database does represent a reliable snapshot of contemporary practice in the four participating centres.
Conclusion
The PRAGMATIC AS survey offers a snapshot of the contemporary management of patients with symptomatic severe AS. Multidisciplinary Heart Teams select an optimal strategy based on age, frailty and comorbidities. Nearly half of patients are sent for TAVI. Only a small minority of patients will not receive valve replacement therapy.
| Impact on daily practice Currently, in selected countries in Europe, multidisciplinary Heart Teams determine an individual’s operative risk and appear to select TAVI as the preferred treatment strategy for elderly patients at moderate to high operative risk. Only a small minority of patients will not receive valve replacement therapy. Competent authorities may need to adjust reimbursement policies and expand to a broader set of patients with severe AS. |
Conflict of interest statement
D. Tchetche and N. Dumonteil are proctors for Edwards Lifesciences, Medtronic and Boston Scientific. B. Marcheix is a proctor for Edwards Lifesciences and Medtronic. P. de Jaegere is a proctor for Boston Scientific and St. Jude. N. Van Mieghem has received research grants from Claret, Boston Scientific, Medtronic and Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data