Abstract
Aims: Transcatheter aortic valve implantation (TAVI) is a new option for patients with severe aortic stenosis at high surgical risk. We compared the clinical outcome of patients referred for TAVI and subsequently treated with TAVI, surgical aortic valve replacement (SAVR), balloon aortic valvuloplasty (BAV), or medical management (MM).
Methods and results: All consecutive patients (n=166, EuroSCORE 24.9±13.9%) referred for TAVI to our two centres were enrolled in a prospective registry and were assigned to SAVR (n=21), TAVI with the CoreValve prosthesis (n=75), BAV (n=20), or MM (n=50) by a multi-specialty team. The primary endpoint was 6-month cardiac mortality. Patients undergoing BAV had a significantly higher EuroSCORE (33.6±15.9%; p=0.01). Median follow-up time was nine months (interquartile range 4.5-12.4 months). Six-month freedom from cardiac death was 81.0±8.6%, 92.0±3.1%, 72.9±10.5%, and 72.7±6.5% for SAVR, TAVI, BAV, and MM groups, respectively. Freedom from major cardiac and cerebrovascular events was 76.2±9.3%, 83.9±4.3%, 72.9±10.5%, and 65.6±6.8% for SAVR, TAVI, BAV, and MM groups, respectively.
Conclusions: With respect to medical management and BAV, TAVI was associated with lower cardiac mortality at six months. Clinical outcome after TAVI was similar to that of less sick patients undergoing SAVR.
Introduction
With the ageing of population in developed countries, calcific aortic stenosis (AS) is a growing health problem, with a prevalence of up to 4% beyond 75 years of age1,2. From the onset of symptoms, AS is usually fatal within two to three years3,4, unless the patient undergoes surgical aortic valve replacement (SAVR), the current gold standard for the treatment of symptomatic AS5,6. However, the risk of surgery is higher in elderly patients with significant comorbidities7,8, and almost one third of patients are not offered surgery4,9.
The recent introduction of transcatheter aortic valve implantation (TAVI) offers a less invasive option for these high-risk patients10,11. Referral of such patients to hospitals offering both TAVI and SAVR represents an unique opportunity to evaluate the results of all currently available opportunities12-15. This prospective registry was designed to compare the clinical outcome of patients evaluated for possible TAVI, and subsequently managed with either TAVI with the CoreValve Revalving System (Medtronic, Minneapolis, MN, USA), or SAVR, or balloon aortic valvuloplasty (BAV), or medical management (MM).
Methods
Patient population
All consecutive patients affected by symptomatic severe AS (aortic valve area ≤1 cm2 or ≤0.6 cm2/m2) referred for possible TAVI to our two centres because of estimated high or prohibitive surgical risk were included in a prospective registry. Surgical risk was estimated by clinical judgement16, and by means of the logistic EuroSCORE, calculated according to published guidelines (http://www.euroscore.org/). All patients were evaluated by a multi-specialty team consisting of cardiac surgeons, interventional and clinical cardiologists, and cardiac anaesthesiologists, and were assigned to SAVR, TAVI, BAV, or MM.
The first step was to assess the feasibility and operative risk of SAVR. Whenever the cardiac surgeons judged that a patient could undergo SAVR with acceptable operative risk, even if the EuroSCORE was ≥15%, traditional surgery was proposed to the patient. Patients who were judged not amenable to SAVR with an acceptable risk were evaluated for TAVI, as well as patients who refused SAVR after being thoroughly informed of the risks and potential benefits of both SAVR and TAVI.
Eligibility for TAVI included either compassionate use, or the presence of the following criteria: age ≥75 years and a logistic EuroSCORE ≥15%, or one or more of the following complicating factors: porcelain aorta, contraindication to open chest surgery, liver cirrhosis, severe chronic obstructive pulmonary disease (COPD, defined as forced expiratory volume in one second <1 L), pulmonary hypertension >60 mmHg, and previous cardiac surgery17. Exclusion criteria for TAVI included: sepsis or active endocarditis; life expectancy <1 year; excessive iliofemoral and subclavian artery disease or tortuosity; aneurysm of the ascending aorta; severe coagulopathy; severe mitral regurgitation; left ventricular ejection fraction <15%; cachexia.
Patients who were unsuitable for both SAVR and TAVI, were considered for BAV. Finally, cachectic patients, patients who refused any intervention, and patients in whom BAV was judged excessively risky, were managed medically.
All patients were followed-up for at least six months by office visit or direct phone contact. The study was approved by the local Ethics Committees, and all participants gave written informed consent.
Surgical aortic valve replacement
SAVR was performed through a standard median sternotomy under cardiopulmonary bypass with moderate (32° C) systemic hypothermia. Myocardial protection was achieved with antegrade and retrograde cold blood cardioplegia. A biological valve was used in all cases. Concomitant coronary artery bypass grafting and/or mitral repair were performed when necessary.
Technique of TAVI and postoperative management
The third generation (18 Fr) CoreValve prosthesis was used in all cases. This prosthesis consists of a trileaflet bioprosthetic porcine pericardial tissue valve, which is mounted and sutured within a self-expanding nitinol stent17. Vascular access was obtained either by percutaneous approach through the common femoral artery, or by surgical cutdown of the subclavian artery. The procedure was performed with the patient under general anaesthesia or local anaesthesia with mild systemic sedative/analgesic treatment, depending on patient collaboration18. Valvuloplasty with a 22 mm or 25 mm balloon under rapid pacing at 180 bpm was performed before CoreValve deployment. The prosthesis was then deployed over a stiff guidewire placed in the left ventricle, under fluoroscopic guidance. Procedural anticoagulation was obtained with weight-adjusted heparin, aiming at an activated clotting time of 250 seconds. Aspirin (100 mg daily) and clopidogrel (300 mg oral load, followed by 75 mg daily) were started at least three days before the procedure; aspirin was continued indefinitely, while clopidogrel was administered for three to six months.
Isolated balloon aortic valvuloplasty
The procedure was performed with the patient under local anaesthesia in combination with a mild systemic sedative/analgesic treatment, depending on patient collaboration. Balloon valvuloplasty was performed through a 12 Fr sheath in the common femoral artery, with an appropriately sized balloon, under rapid pacing.
Medical therapy
Patients excluded from any intervention received the appropriate medical therapy, and were referred back to the proposing physician.
Endpoints and definitions
The primary endpoint was cardiac mortality at 6-month follow-up. All deaths were considered cardiac-related unless a non-cardiac origin could be clearly established by clinical and/or pathological study. The secondary endpoints were overall mortality and a composite of major cardiovascular and cerebrovascular events (MACCE), consisting of death from any cause, myocardial infarction, and stroke, whichever occurred first during six months of follow-up. The diagnosis of myocardial infarction was based on either the development of new pathological Q waves in ≥ 2 contiguous electrocardiographic leads, and/or an elevation of CK-MB isoenzyme > 2 times the upper limit of normal.
Furthermore, we evaluated the technical and procedural success for SAVR and TAVI, and the quality of life at follow-up. Technical success for TAVI was defined as adequate technical placement of the device within the aortic root, as described by Piazza19. Procedural success was defined as technical success without major acute complications (death, stroke, myocardial infarction, and procedural complications) during hospital stay. Procedural complications of TAVI included aortic root dissection, left or right ventricular perforation, cardiac tamponade, urgent conversion to open-chest surgery, and major bleeding, defined as haemorrhage requiring vascular surgery and/or transfusion of ≥2 blood units. Quality of life was evaluated in terms of re-hospitalisation for cardiac causes, New York Heart Association (NYHA) functional class, and loss of ability to self-care at follow-up20.
Statistics
Categorical variables were presented as frequencies and were compared by chi-square or Fisher exact test, as appropriate. Continuous variables not normally distributed were presented as median and interquartile range. Comparison among groups was performed with Kruskal-Wallis one-way analysis of variance (ANOVA). Multiple comparison between groups was performed with Bonferroni’s test. Actuarial freedom from adverse events was assessed with Kaplan-Meier method. A p value < 0.05 was considered statistically significant. Statistical tests were performed with StatXact 9 (Cytel Inc, Cambridge, MA, USA) software.
Results
Patient population
One hundred and sixty-six (166) consecutive patients screened for TAVI from September 2007 to February 2009 were included in this prospective cohort study (Figure 1). Of these, 38 (22.9%) were considered eligible for SAVR, but only 21 (12.7%) gave their consent to SAVR. The remaining 128 patients were excluded from surgery because of the following reasons: age ≥75 years and EuroSCORE >15% in 96 cases (57.8%), EuroSCORE ≤15% in the presence of severe comorbidities (mainly severe COPD and end-stage renal failure) in 19 cases (11.4%), porcelain aorta in nine cases (5.4%), and previous mediastinum radiotherapy in four cases (2.4%). These 128 patients and the 17 patients who refused SAVR were evaluated for the technical feasibility of TAVI, and 45 of them were excluded from TAVI because of the following reasons: concomitant severe mitral regurgitation in 11 cases (6.6%), aortic annulus diameter <19 mm or >27 mm in 10 cases (6.0%), cachexia in six cases (3.6%), progressive disease with life expectancy <1 year in five cases (3.0%), severe left ventricular dysfunction with an ejection fraction <15% in five cases (3.0%), excessive femoral and/or subclavian tortuosity and calcification in five cases (3.0%), and severe thrombocytopenia in three cases (2.1%). Of these 45 patients unsuitable for TAVI, 20 underwent BAV (12.0%), while 25 remained on medical therapy because of cachexia in 10 cases (6.0%), excessive risk even for BAV in six cases (3.6%), refusal of any intervention in five cases (3.0%), and lack of any arterial access in four cases (2.4%). Finally, of the 100 patients suitable for TAVI, 25 refused to undergo any interventional procedure and were managed medically, and 75 underwent TAVI. In summary, 21 patients (12.6%) underwent SAVR, 75 (45.2%) underwent TAVI, 20 (12.0%) underwent BAV, and 50 (30.1%) did not undergo any intervention.
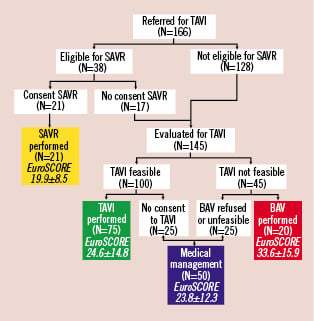
Figure 1. Flow chart of clinical follow-up of patient population. BAV: balloon aortic valvuloplasty; MM: medical management; SAVR: surgical aortic valve replacement; TAVI: percutaneous aortic valve replacement
Baseline characteristics of the patients are reported in Table 1. Patients undergoing BAV had a significantly higher median logistic EuroSCORE (32.3; interquartile range 21.2-45.9) compared with SAVR (17.0; interquartile range 15.0-26.1; p=0.009), and MM patients (21.0; interquartile range 14.0-30.9; p=0.04), but not with TAVI patients (21.9; interquartile range 14.8-33.0, p=0.06). Furthermore, BAV patients were more frequently in NYHA class III-IV (95.0%) compared with SAVR (57.1%), TAVI (60.0%), and MM patients (52.0%; p=0.009).
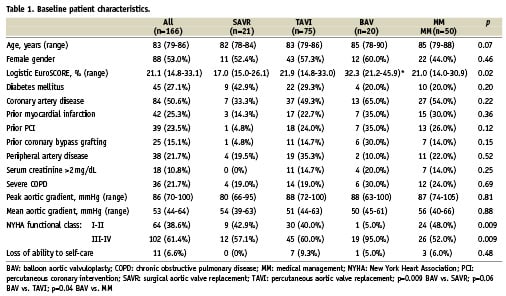
The median aortic valve area index was 0.30 cm2/m2 (interquartile range 0.24-0.40 cm2/m2) and the median mean aortic gradient was 53 mmHg (interquartile range 44-64 mmHg), without significant differences among groups.
In-hospital results
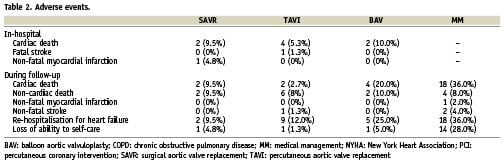
Technical success was 100% in both SAVR and TAVI groups. All patients in the SAVR group received a porcine bioprosthesis. Four SAVR patients (19.0%) had concomitant coronary artery bypass grafting, and one (4.8%) mitral valve repair. Procedural success was 85.7% for SAVR and 93.3% for TAVI. There were four in-hospital deaths after TAVI (5.3%), three because of cardiogenic shock and one because of multi-organ failure, and two after SAVR (9.5%), because of cardiogenic shock (Table 2). One patient suffered fatal stroke after TAVI (1.3%), and one suffered myocardial infarction during SAVR (4.8%). Overall in-hospital MACCE rate was 14.3% for SAVR and 6.7% for TAVI (p=0.37). Additional periprocedural complications of TAVI were major bleeding in two patients (2.7%), and vascular access site injury in three (4.0%). In-hospital mortality of BAV was 10.0%.
Six-month clinical outcome
The 6-month follow-up completeness was 94.0% (10 patients were lost to follow-up, three in both MM and BAV groups, two in both SAVR and TAVI groups). Median follow-up time was 8.5 months (interquartile range 4.5-12.4 months). Table 2 summarises the main adverse events occurring during follow-up.
A Kaplan-Meier curve of freedom from cardiac death was generated for all treatment groups (Figure 2). In particular, 6-month freedom from cardiac death was highest for TAVI patients (92.0±3.1%), with respect to MM patients (72.7±6.5%), BAV patients (72.9±10.5%), and SAVR patients (81.0±8.6%).
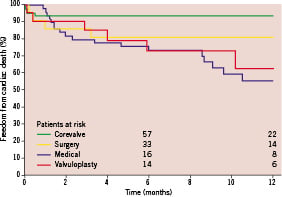
Figure 2. One-year survival Kaplan-Meier estimates of freedom from cardiac death in patients assigned to TAVI, SAVR, BAV, and MM. BAV: balloon aortic valvuloplasty; MM: medical management; SAVR: surgical aortic valve replacement; TAVI: percutaneous aortic valve replacement
Kaplan-Meier estimates of overall survival are depicted in Figure 3. In particular, 6-month survival was higher for TAVI patients (83.9±4.3%) and SAVR patients (81.0±8.6%), and lower for MM patients (65.6±6.8%) and BAV patients (72.9±10.5%).
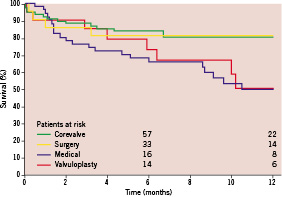
Figure 3. One-year Kaplan-Meier estimates of survival in patients assigned to TAVI, SAVR, BAV, and MM. BAV: balloon aortic valvuloplasty; MM: medical management; SAVR: surgical aortic valve replacement; TAVI: percutaneous aortic valve replacement
Finally, a Kaplan-Meier curve of freedom from MACCE was generated for all treatment groups (Figure 4). Patients undergoing TAVI showed the best outcome (83.9±4.3%) compared with MM patients (65.6±6.8%), BAV patients (72.9±10.5%), and SAVR patients (76.2±9.3%).
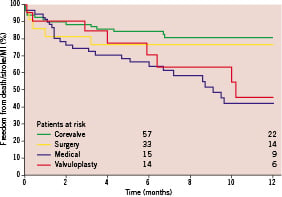
Figure 4. One-year survival Kaplan-Meier estimates of freedom from major adverse cardiac and cerebrovascular events (MACCE) in patients assigned to TAVI, SAVR, BAV, and MM. BAV: balloon aortic valvuloplasty; MM: medical management; SAVR: surgical aortic valve replacement; TAVI: percutaneous aortic valve replacement
Concerning quality of life at 6-month follow-up, MM and BAV patients showed a significantly higher rate of re-hospitalisation because of heart failure, compared with both TAVI and SAVR patients, (36.0%, 25.0%, 12.0%, and 9.5%, respectively; p=0.006). In addition, loss of ability to self-care was significantly higher in MM patients vs. BAV, TAVI, and SAVR patients (28.0% vs. 5.0%, 1.3%, and 4.8%, respectively; p=0.0001). Finally, also the rate of NYHA class III-IV was markedly worse in MM patients vs. BAV, TAVI, and SAVR patients (36.0% vs. 15.0%, 0%, and 9.5%, respectively; p=0.0001).
Discussion
The major finding of our study was that the majority of the elderly patients with AS and multiple comorbidities referred for TAVI could be treated by either TAVI (45.2%) or SAVR (12.7%) with excellent procedural results and favourable 6-month clinical outcome. On the other hand, patients who remained on medical therapy or who underwent isolated BAV showed a markedly worse cardiac mortality and quality of life.
Surgical aortic valve replacement in the elderly
Currently, SAVR remains the gold standard treatment for symptomatic severe AS5,6. However, as the surgical risk is definitely higher in elderly patients, particularly when significant comorbidities are present7,8, many patients are not referred for SAVR, although their prognosis on medical therapy is dismal. In the Euro Heart Survey published in 2005, 33% of patients with symptomatic severe valve disease were not referred for surgery9. Other studies have similarly shown that 27% to 41% patients with severe symptomatic AS do not undergo SAVR3,4.
Early reports of SAVR in elderly patients showed high operative mortality rates21; however, more recent reports have shown a mortality rate of 2-10% for isolated SAVR in selected populations with few comorbidities8,22. In our very high-risk population, in the hands of experienced surgeons, an acceptable 10% in-hospital mortality after SAVR was obtained.
Balloon aortic valvuloplasty
For several decades, BAV has been more a palliative treatment rather than a real alternative to SAVR for patients with very high or prohibitive surgical risk. The hospital mortality for BAV ranges from 3 to 10%, with a hospital morbidity of 10 to 25%23,24. Furthermore, immediate restenosis occurs in 25% of patients, and in 66% within six months23. In the present registry, we performed BAV in the most symptomatic, clinically unstable patients, with the highest EuroSCORE (median 32.3; interquartile range 21.2-45.9). Accordingly, their 6-month survival was low, and similar to that of patients not undergoing any intervention (78.9±9.4 vs. 67.8±6.6, respectively). This finding confirmed that BAV did not significantly modify patient prognosis, allowing only for a variable degree of transient symptomatic improvement15. In our initial experience here described, BAV was reserved to patients technically unsuitable for both SAVR and TAVI, and was not considered as a bridge to TAVI. Later on, we began to perform BAV in low-gradient, low-ejection fraction patients, followed by TAVI (usually within two months of BAV) in those patients who showed some degree of recovery of left ventricular function after BAV. Thus, in our opinion, BAV should currently be regarded mainly as a bridge to a more effective treatment by means of TAVI (or, rarely, SAVR) in selected low-gradient, low-output aortic stenosis patients, as it does not improve prognosis when it is performed as an isolated treatment.
Transcatheter aortic valve implantation
The recent introduction of TAVI opened new perspectives for the treatment of patients at very high surgical risk, and also of patients who refuse to undergo open-chest surgery, but who accept a less invasive approach. The procedural performance and 30-day outcomes of a large cohort of high-risk patients undergoing TAVI using the third generation CoreValve bioprosthesis have been recently reported19. At 30 days, the all-cause mortality rate was 8%, and the combined rate of death, stroke, and myocardial infarction was 9.3%. Similarly, TAVI with the Edwards SAPIEN prosthesis has also been reported to yield excellent short- and medium-term results, with an actuarial survival of 88.7%, 73.8%, and 60.9% at 1, 12, and 24 months, respectively25.
Tertiary care hospitals providing facilities for both SAVR and TAVI offer a unique opportunity to evaluate the clinical outcome of the heterogeneous population of patients with severe AS who are referred for TAVI12-15. All previous papers reporting the outcome of patients referred for TAVI with the CoreValve12,13 or with the SAPIEN prosthesis14,15 described higher 6-month mortality for patients treated with BAV or MM, with respect to patients undergoing TAVI or SAVR. In particular, Otten and co-workers reported that patients who refused any treatment had the highest surgical risk and the highest 1-year mortality.
Our experience confirmed the safety and efficacy of TAVI in high-risk patients, with encouraging 6-month freedom from cardiac death (92.0±3.1%) and from MACCE (83.9±4.3%). Interestingly, the survival curves of patients treated with TAVI and of patients remaining on MM crossed just beyond one month, suggesting that the procedural risk of TAVI was counterbalanced by the benefit on mortality very early after CoreValve implantation. In addition, both in-hospital and 6-month mortality of TAVI were similar to those of SAVR, despite the higher logistic EuroSCORE. However, this registry was not designed to compare the outcomes of TAVI vs. SAVR, and our findings should not be generalised.
In this prospective registry, the choice of treatment for each patient was taken by consensus among physicians of different specialties, including cardiac surgeons, interventional and clinical cardiologists, and cardiac anaesthesiologists. The importance of such agreement was demonstrated by the fact that 12.7% of the patients referred for TAVI actually underwent SAVR. This finding outlines that the choice of treatment in this selected population of patients cannot rely just on surgical risk score assessment or on the application of protocols. As recently demonstrated by Piazza and co-workers, both the EuroSCORE and the Society of Thoracic Surgeons Predicted Risk of Mortality (STS) score have suboptimal discriminatory power and calibration, when applied to patients undergoing TAVI with the CoreValve26. Currently, clinical judgment and the multidisciplinary approach remain the mainstay of clinical decision making.
Quality of life
Both TAVI and SAVR obtained a marked benefit of on the quality of life at 6-month follow-up, compared with medically managed patients, with regards to re-hospitalisation because of cardiac causes (p=0.005), NYHA class (p=0.0001), and loss of ability to self care (p=0.0001). These results are particularly relevant, given the limited life expectancy of elderly patients with severe aortic stenosis.
Limitations
Our registry suffers from all the limitations of non-randomised comparisons between different treatment options, and from the imbalance in the baseline clinical profile among groups. However, it offers a real-world portrait of how TAVI may impact on the outcome of elderly patients with AS and relevant comorbidities, at high risk for SAVR. Another limitation is the relatively short follow-up duration, although significant differences in clinical outcome could be already detected at six months.
Conclusions
The population of patients referred for possible TAVI consists of really high-risk subjects with multiple severe comorbidities. Medical management alone was associated with a high mortality rate, substantially unaffected by BAV. Careful patient evaluation by a multi-specialty team allowed for the selection of those patients who could successfully undergo TAVI or SAVR. Both interventions showed a positive impact on 6-month clinical outcome and quality of life of the patients.

