
Abstract
Aims: The indication for transcatheter aortic valve implantation (TAVI) has evolved from inoperable patients to patients at increased surgical risk. In low-risk patients, surgical aortic valve replacement (SAVR) remains the standard of care. The aim of this study was to explore the outcomes of TAVI and SAVR in patients with a Society of Thoracic Surgeons (STS) predicted risk of mortality (PROM) score below 3% in the SURTAVI trial.
Methods and results: In SURTAVI, patients at intermediate surgical risk based on Heart Team consensus were randomised to TAVI or SAVR. We stratified the overall patient population into quintiles based on the STS PROM score; the one-year mortality was correlated with the mean STS PROM score in each quintile. The quintiles were regrouped into three clinically relevant categories of STS score: less than 3%, 3 to <5%, and >5%. All-cause mortality or disabling stroke in each risk stratum was compared between TAVI and SAVR. Linear regressions between mean values of STS PROM in each quintile and observed all-cause mortality at one year showed great association for the global population (r2=0.92), TAVI (r2=0.89) and SAVR cohorts (r2=0.73). All-cause mortality or disabling stroke of TAVI vs. SAVR was 1.5% vs. 6.5% (p=0.04), 6.5% vs. 7.6% (p=0.52) and 13.5% vs. 11.0% (p=0.40) in the <3%, 3-5%, and ≥5% STS score strata, respectively.
Conclusions: Among patients at intermediate surgical risk but with an STS PROM <3%, TAVI may achieve superior clinical outcomes compared to SAVR. These findings support the need for an adequately powered randomised trial to compare TAVI with SAVR in patients at low operative risk.
Abbreviations
BiPAP: bi-level positive airway pressure
FEV1: forced expiratory volume in 1 second
PROM: predicted risk of mortality
SAVR: surgical aortic valve replacement
SPAP: estimated systolic pulmonary artery pressure
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
VARC-2: Valve Academic Research Consortium-2
Introduction
The comparisons between TAVI and SAVR indicate favourable results for TAVI at higher Society of Thoracic Surgeons (STS) predicted risk of mortality (PROM) but appear similar among patients with STS PROM below 6-8%1. For patients with increased surgical risk, non-inferiority and even superiority of TAVI compared with SAVR has been demonstrated consistently1-5. However, a recent STS database survey including 113,377 patients with a mean age of 65.3±13.0 years and an average STS score of 1.67±0.94% indicates that 80% of all SAVR patients are at low risk for SAVR based on an STS score of <4%6. Clearly this data set is different from the patients who have been included in randomised TAVI trials to date. Furthermore, the STS score was designed and validated for risk stratification and prediction of 30-day mortality after cardiac surgery in general7 and appeared not to be calibrated in TAVI cohorts where it typically overestimates procedural risk8.
Since TAVI originated in patients who were inoperable or at high risk for SAVR, the criteria for risk stratification were naturally borrowed from the surgical field. However, since the procedure was performed largely in an elderly population, it was rapidly recognised that additional risk factors summarising aspects of frailty were also predictors of mortality8-12. Therefore, decision making for TAVI currently takes into consideration not only the STS PROM but also other parameters of the cumulative risk burden that are not included in the STS score or logistic EuroSCORE calculation12,13.
Elderly patients who are considered for TAVI often have risk variables that are not considered in the STS model. Unsurprisingly, Heart Teams may judge a patient to be at elevated operative risk despite having a calculated STS score of <3%.
The aim of this post hoc analysis of the SURTAVI randomised trial was to compare clinical outcome of TAVI vs. SAVR in patients with an STS score of <3%.
Methods
SURTAVI was a non-inferiority, multicentre, randomised clinical trial designed to test the safety and efficacy of SAVR and TAVI in patients with severe and symptomatic aortic stenosis considered to be at intermediate operative risk. Subjects considered eligible were enrolled at 87 centres and were randomly allocated in a 1:1 ratio to receive TAVI with the use of a self-expanding bioprosthesis or undergo surgery. The details of the trial have been described elsewhere13. A total of 1,746 subjects were randomised; 1,660 patients underwent an attempted procedure and composed the modified intention-to-treat analysis cohort. Patients were divided into three strata based on their STS PROM: <3%, 3-5%, ≥5%. The primary endpoint was a composite of all-cause mortality or disabling stroke.
RISK STRATIFICATION AND FUNCTIONAL ASSESSMENT
The Society of Thoracic Surgeons predicted risk of mortality (STS PROM) calculator was used for operative mortality risk estimation7. In order to include patients with a predicted operative mortality of 3-15% as estimated by the local Heart Team, a combination of the conventional risk scores and a qualitative assessment of the cumulative clinical risk (determined by a list of risk factors not captured by the STS score) was used12.
However, the Heart Team was free to weigh each of the following risk factors: (i) respiratory disease severity –forced expiratory volume in one second (FEV1) 750-1,000 cc or FEV1 <750 cc, home oxygen therapy, and bi-level positive airway pressure (BiPAP); (ii) estimated systolic pulmonary artery pressure (SPAP) 60-80 mmHg or >80 mmHg; and (iii) other risk factors such as elevated BNP ≥550 pg/ml or NT pro-BNP ≥3,200 pg/ml, 5-metre gait speed ≥6 seconds, severe diastolic dysfunction, liver cirrhosis, and severe aortic calcification. Physical activity and independence were systematically assessed in all patients (by means of the 5-metre gait speed test and the Katz index, respectively).
TRIAL ENDPOINTS
The SURTAVI trial primary endpoint was a composite of death from any cause or disabling stroke at 24 months13. One-year outcomes reported here include the composite outcome as well as the pre-specified secondary endpoints including major adverse cardiovascular and cerebrovascular events (MACCE), which consisted of death from any cause, myocardial infarction, all types of stroke and any reintervention, and encephalopathy. Disabling stroke was defined according to the criteria of the Valve Academic Research Consortium-2 (VARC-2)14. All patients were seen by a neurologist or stroke specialist, and neurologic events were adjudicated by a neurologist on the clinical events committee. Encephalopathy was defined in the protocol as an altered mental state (seizures, delirium, confusion, hallucinations, dementia, coma, psychiatric episode).
STATISTICAL ANALYSIS
All of the 1,660 patients in the modified intention-to-treat analysis cohort are included in this analysis and have reached one year of follow-up. Calibration between observed and expected mortality at 30 days based on STS PROM was investigated in quintiles of the SAVR arm, the TAVI arm and the pooled population (Supplementary Table 1). Similarly, all-cause mortality observed at one year was compared to the predicted mortalities at 30 days; this statistical and epidemiological practice, which is at variance with the original concept of the STS PROM score, has been reported previously in the literature15. A linear regression model was fitted between observed all-cause mortality at one year and mean values of STS PROM score in each quintile for each of the three populations; the intercept, slope and R square from the model result were reported. We also performed logistic regression analysis to assess the predictive value of the STS PROM score for 30-day and one-year all-cause mortality. The goodness of fit was evaluated using the Hosmer-Lemeshow test, and calibration and discrimination were assessed by c-statistics, the Brier score and Somers’ D test (Supplementary Table 1).
The next analytical step in the clinical evaluation of these quintile assessments was to regroup the five equally populated cohorts into three strata of risk categorised by a single digit number of STS PROM score (thereby creating groups of unequal extent, at variance with the balanced quintile subdivision). Rounding the STS percentage and standard deviation found in the quintiles, a consensus emerged among the investigators that the STS group of criteria less than 3%, 3% to less than 5%, and 5% or more than 5% would be highly relevant from a clinical point of view.
Baseline demographic and clinical characteristics were compared within each STS stratum between SAVR and TAVI and among the overall population with the analysis of variance (ANOVA) test, and pairs of subcategories from the pooled population. Continuous data are presented as mean±standard deviation and were compared using the Student’s t-test or ANOVA, as appropriate. Categorical variables were compared using Fisher’s exact test or the chi-square test, as appropriate. Time-to-event analysis was performed using the Kaplan-Meier method, while comparison between the groups was carried out using the log-rank test. Statistical analysis was performed with SAS software, version 9.2 (SAS Institute, Cary, NC, USA), and a two-tailed p<0.05 defined the statistical significance.
Results
The STS PROM of the quintile cohorts in the entire randomised population was 2.4±0.6%, 3.6±0.3%, 4.3±0.2%, 5.1±0.3% and 6.8±1.0%, respectively (Supplementary Table 2). The STS PROM did not predict mortality at 30 days for SAVR or TAVI. In the surgical cohort, the expected mortality at 30 days was systematically and substantially overestimated when compared to the observed mortality (Supplementary Table 2, Figure 1).
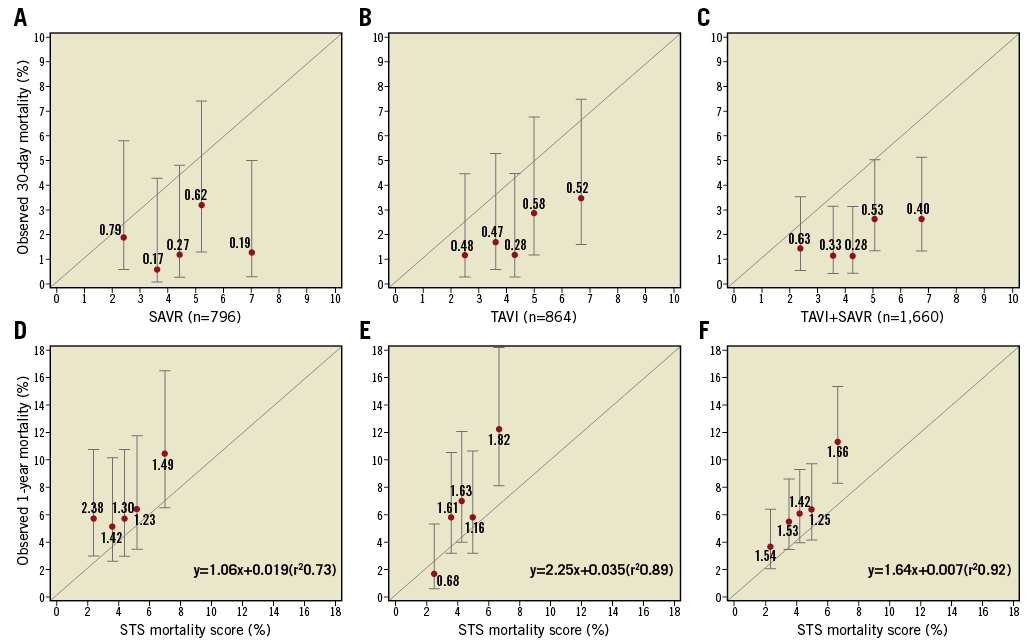
Figure 1. Estimated Kaplan-Meier 30-day and one-year mortality in the SAVR, TAVI and combined cohorts of patients, divided into quintiles. A), B), & C) The observed 30-day mortality in the quintiles of the SAVR, TAVI and combined cohorts, respectively. Each dot stands for the 30-day Kaplan-Meier rate with associated 95% CI. The number by the dot is the O/E ratio (observed 30-day Kaplan-Meier rate/mean STS score). D), E), & F) The observed one-year mortality in the quintiles of the SAVR, TAVI and combined cohorts, respectively. Each dot stands for the one-year Kaplan-Meier rate with associated 95% CI. The number by the dot is the O/E ratio (observed one-year Kaplan-Meier rate/mean STS score).
When the STS PROM score of the pooled population (SAVR+TAVI) was correlated with the observed mortality at one year, a significant linear correlation (y=–0.68+1.64x[r2=0.92]) was observed (Figure 1). Supplementary Table 2 shows the mortality at one year ranging from 3.7% in the lowest quintile to 11.3% in the highest quintile of the pooled population (n=1,660).
The observed mortality at one year in each quintile of the entire population was higher than the mortality expected at 30 days, with the observed/expected (O/E) ratios of 1.54, 1.53, 1.42, 1.25 and 1.66, respectively (Figure 1).
In the SAVR arm, a similar observation was made, with the exception of the lowest quintile for which the O/E ratio was 2.38 (Figure 1). In the TAVI arm, the linear regression had a steeper slope than the line of identity between the observed and the expected mortality, and noticeably the O/E ratio in the lowest quintile was 0.68 (Figure 1).
The use of the traditional and objective STS score to subdivide the whole randomised population into three strata of progressive risk of mortality generates in each stratum two cohorts of patients randomised either to TAVI or SAVR with 31 comparable baseline characteristics16.
In the cohort of patients with an STS score of <3%, the sole significant difference between the TAVI and SAVR cohorts was the medical history of prior TIA (11.5%, 15/131 versus 4.1%, 5/123, p-value 0.0290). All the other parameters were comparable (Supplementary Table 3). In the stratum of STS score 3-5%, one significant difference was observed in the 6-minute walk test - SAVR (271±117 metres) vs. TAVI (253±115 metres). In the STS score stratum ≥5%, one parameter in the medical history (congestive heart failure) differed significantly (p-value 0.0092) between the TAVI treatment arm (96.4%, 244/253) and the SAVR treatment arm (99.6%, 267/268).
When comparing the baseline characteristics of the subjects among the three STS strata, there was an increase of age and comorbidities such as diabetes, peripheral vascular disease, coronary artery disease (with prior CABG) and heart failure with NYHA class of more than II (Supplementary Table 4).
The primary and secondary outcomes at one year are shown in Table 1. The Kaplan-Meier curves for the primary endpoint in both groups are shown in Figure 2. In the less than 3% STS score stratum, the primary outcome of all-cause death or disabling stroke was significantly lower in the TAVI than in the SAVR arm (1.5% vs. 6.5%, p=0.0421; Kaplan-Meier method). The Kaplan-Meier curves for both groups are shown in Figure 2. In the other two strata, primary outcomes were comparable (Table 1). In the three strata, the differences in all-cause mortality alone or itemised causes of death did not reach conventional levels of statistical significance. Similarly, in none of the three strata did the difference in disabling stroke reach a significant statistical level. With the exception of the strata with an STS score of <3%, TAVI was associated with a significantly higher vascular complication rate. The rate of permanent pacemaker implantation was significantly higher in the TAVI population. SAVR treatment was complicated by significantly higher rates of kidney injury, overall bleeding, atrial fibrillation and encephalopathy in the two risk strata superior to 3%. Overall, neurological events had a tendency to be more frequent in the SAVR group in the stratum of <3% (p=0.072), were significantly higher in the stratum of 3% to less than 5% (p=0.0028), and were comparable in the stratum of ≥5%.
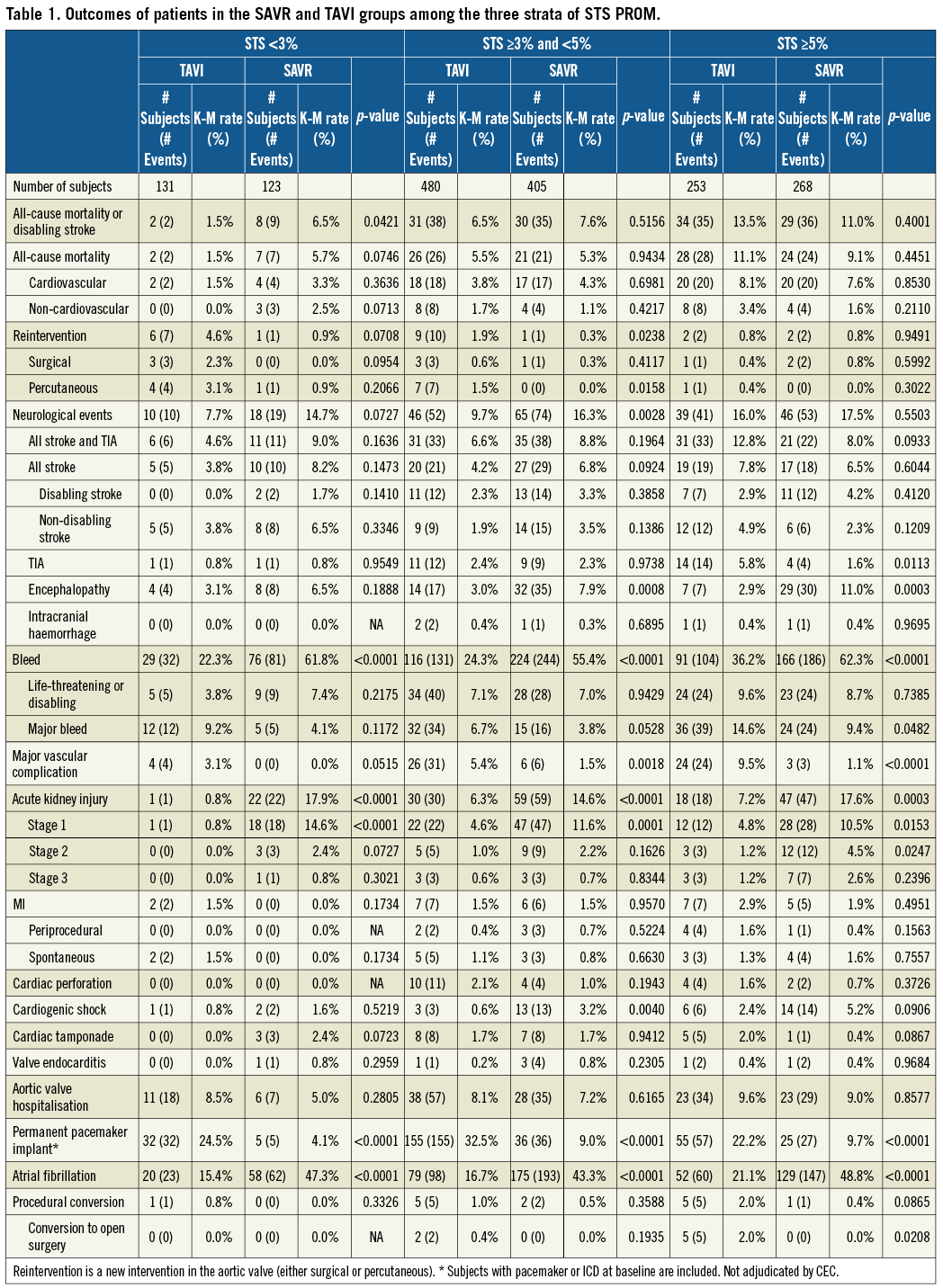
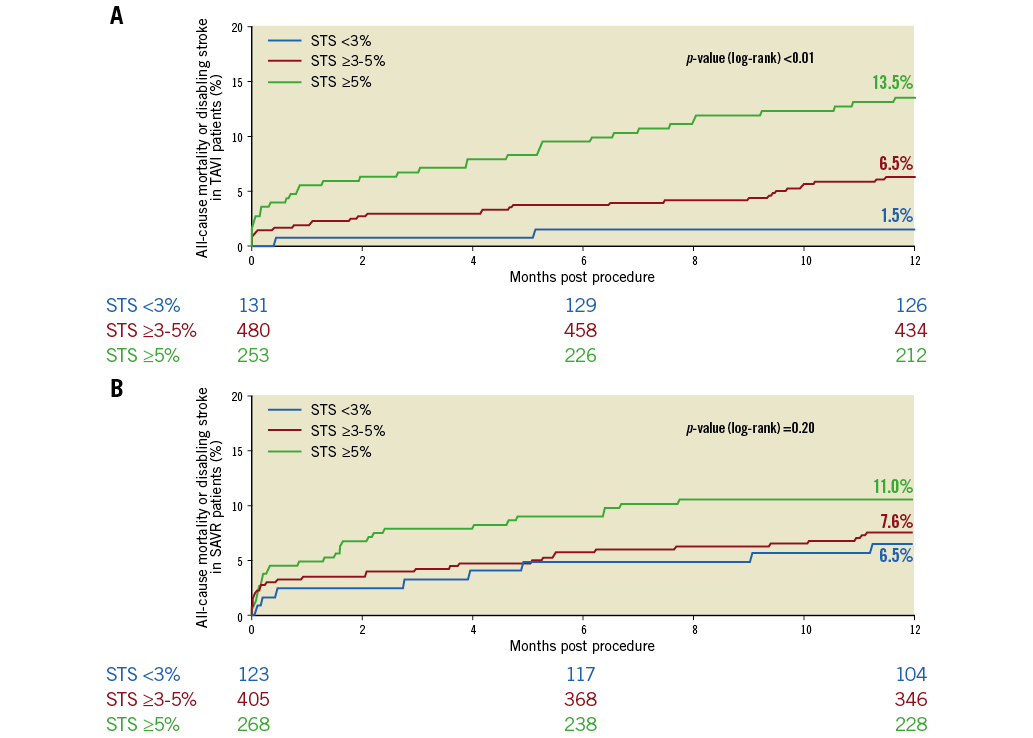
Figure 2. Primary endpoint at one year for patients randomised to TAVI or SAVR, divided among the different strata of STS PROM scores. Kaplan-Meier curves are shown for STS <3% (blue), STS ≥3% and <5% (red) and STS ≥5% (green). A) Time-to-event graph for patients undergoing TAVI. B) Time-to-event graph for patients undergoing SAVR.
Discussion
The major findings of this post hoc analysis of the SURTAVI trial are:
1) STS PROM does not predict the 30-day mortality of patients treated with either TAVI or SAVR but does correlate with one-year mortality.
2) All-cause mortality at one year in the population of the SURTAVI trial appears to correlate with the estimated mortality at 30 days (STS PROM) in TAVI and SAVR, although the O/E relationship differs between the two modalities of treatment in each quintile.
3) In patients with an STS PROM of less than 3%, the primary composite endpoint of all-cause mortality or disabling stroke is higher with SAVR than with TAVI (p-value 0.0421).
The present analysis investigated the largest randomised cohort of patients at lower operative risk defined by an STS score of <3%. TAVI appeared to be safer than SAVR, particularly in patients with the lowest STS score.
Recently, Tarantini et al discussed the definition of risk and analysed evidence from randomised trials and registries in lower-risk patients17. The analysis, which reviewed PARTNER 2, SURTAVI, NOTION, S3i vs. PARTNER 2 surgical cohort propensity analysis15,18-26 and numerous European registries, emphasised that the surgical score significantly overestimated TAVI mortality at 30 days.
Since no specific score existed for risk assessment before TAVI, the widely used surgical ones were naturally used for this purpose. With our findings we can also speculate that the decision for aortic valve replacement (surgical or percutaneous) should take into account not only the numerical risk score of the patient (e.g., EuroSCORE, STS PROM), but a combination of factors that might confer on the patient more or less risk12,13.
When clinical outcomes of the less than 3% risk stratum in the SURTAVI trial were compared to data from other randomised patients at low and intermediate risk, it appears that our cohort of patients has the lowest mean STS PROM and logistic EuroSCORE when compared to all the other RCTs, whereas the less than 3% STS PROM cohort of SURTAVI at one year had the best clinical results in terms of all-cause mortality, disabling stroke and major vascular complication (Supplementary Table 5, Supplementary Table 6).
Interestingly, when focusing on the neurological events, we can see a clear trend towards a decrease in the occurrence of all stroke after TAVI in the lower-risk surgical group patients. On the other hand, after SAVR we also see a trend towards increasing all stroke in the lower-risk groups. At a first glance, our findings hold a certain appeal to perform TAVI in the lower-risk group of patients. If, on the one hand, patients at lower risk undergoing TAVI have a higher need for permanent pacemaker (PPM) implantation compared with SAVR, on the other hand they experience significantly lower incidences of new-onset atrial fibrillation, bleeding and acute kidney injury.
This finding mandates a trial dedicated to low-risk patients. Based on the observed primary endpoint in the less than 3% STS PROM stratum, the sample size calculation would require at least 400 patients per group with a 95% power and an alpha error of less than 0.05. Currently, there is a trial in the recruitment phase (NCT02701283) with that approach – enrolling 1,200 patients with a predicted risk for SAVR of less than 3%.
Limitations
The first limitation is that this analysis is post hoc and not pre-specified. The categorical subdivision into risk groups with an STS PROM score of less than 3%, 3% to less than 5%, and 5% and more than 5% is pragmatic but arbitrary, although relying on a linear correlation between STS PROM and all-cause mortality. Secondly, Heart Team assessment of surgical risk assimilates objective aggregate risk indicators based on comorbidities, incremental risk factors not included in risk assessment tools, and an often subjective assessment of frailty. Although the objective data around frailty presented in this manuscript (grip strength, gait speed, and BMI <21 kg/m2) do not indicate an enrichment of frail patients in the STS <3% subgroup, it is possible that patients with an STS score indicative of low surgical risk were often deemed by the Heart Team to be at intermediate risk specifically due to frailty.
All-cause mortality, that does not need any adjudication, is the ultimate criterion of clinical outcome assessment in a randomised population submitted to comparative treatment, but it has to be considered that all-cause mortality lacks specificity, in particular in the elderly. This report is obviously a hypothesis-generating analysis, that can only be tested and verified in an adequately powered trial using the identical criterion of an STS PROM score of <3%.
Conclusions
When compared to SAVR with an STS score of less than 3%, TAVI in the context of a randomised trial could achieve a superior primary endpoint, traditionally based on all-cause death or disabling stroke but would require a prospective, adequately powered trial using specifically the inclusion criterion of an STS PROM score of less than 3%.
| Impact on daily practice With the improvement in transcatheter aortic valve technology and the expansion of TAVI indications, knowledge of the outcomes in patients with a low STS PROM score is awaited. In this sub-analysis of the SURTAVI randomised clinical outcome trial, we show that TAVI may present lower mortality or disabling stroke than SAVR at one year in this group of patients. Randomised clinical trials designed specifically for this group are currently underway. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Funding
SURTAVI was funded by Medtronic.
Conflict of interest statement
M. Reardon is a member of the advisory board of Medtronic. J. Popma and H. Amrane declare institutional grants received from Medtronic. N. Kleiman has received research grants and compensation for educational services from Medtronic. P. Serruys has received personal fees from Medtronic. Y. Chang and A.P. Kappetein are employees of Medtronic. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
The complete list of references can be found in the online version of this paper.
Supplementary data
Supplementary Table 1. Scores for the goodness of fit (Hosmer-Lemeshow test) and for calibration and discrimination (c-statistics, Brier score and Somers’ D test) for STS PROM in both groups of patients (SAVR and TAVI) and for the whole population (SAVR+TAVI).
Supplementary Table 2. Observed (Kaplan-Meier estimates) at 30 days and one year and expected mortality (STS PROM) in the quintiles of STS PROM scores for patients in the whole randomised population (SAVR+TAVI) and for the separate groups of SAVR and TAVI.
Supplementary Table 3. Baseline characteristics of patients in the SAVR and TAVI groups among the three different strata of STS PROM.
Supplementary Table 4. Baseline characteristics of patients in the whole randomised population among the three different strata of STS PROM.
Supplementary Table 5. Characteristics of design, patients and interventions by treatment group of the low- to intermediate-risk TAVI vs. SAVR studies.
Supplementary Table 6. Single-digit endpoints of studies comparing TAVI vs. SAVR in low- to intermediate-risk patients.
To read the full content of this article, please download the PDF.

