
During the past decade, several important clinical trials have provided evidence supporting a paradigm shift from surgical aortic valve replacement (SAVR) towards transcatheter aortic valve implantation (TAVI) for the treatment of patients with symptomatic severe aortic valve stenosis (AS). Randomised controlled trials (RCTs) are the cornerstone of evidence-based medicine and have been the major driver of this paradigm shift. However, other study types such as international multicentre registries (both prospective and retrospective), post-market real-world surveillance studies, and head-to-head comparisons of different types of transcatheter heart valve (THV) are also needed in order to strengthen this evidence and provide insights into the care of patients who are underrepresented in RCTs (Figure 1).
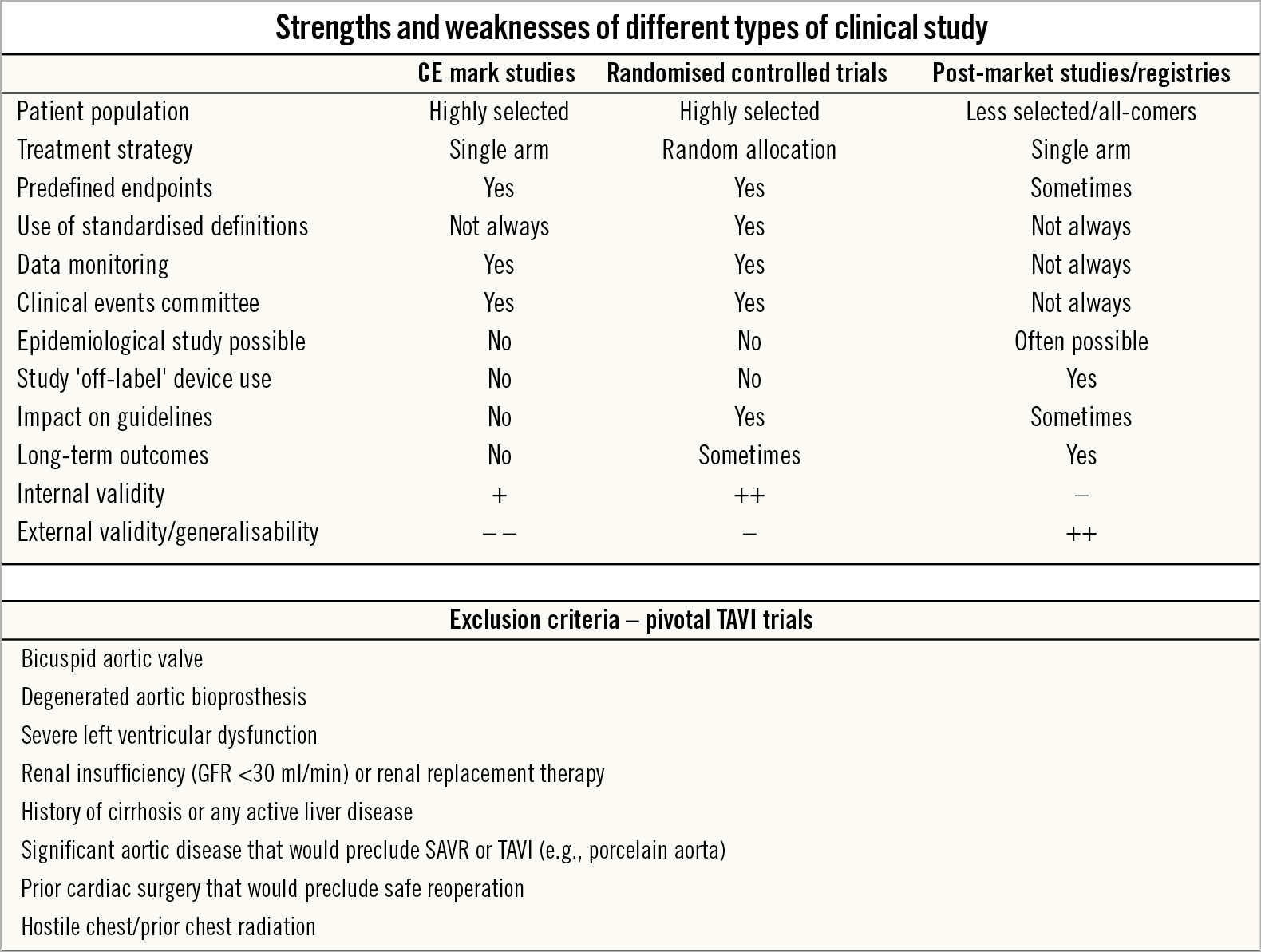
Figure 1. Comparison of different TAVI study types. Overview of the different strengths and weaknesses of different types of clinical TAVI study. A significant number of patients are excluded from the pivotal TAVI trials but are included in real-world registries. GFR: glomerular filtration rate; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation
Randomised controlled trials
The pivotal PARTNER1,2,3, CoreValve/Evolut4,5,6 and NOTION7 trials comparing TAVI with SAVR have provided a comprehensive picture with patients ranging from high to low surgical risk. Based on these RCTs, authorities such as the European Agency for the Evaluation of Medical Products (EMEA) and the Food and Drug Administration (FDA) have expanded the indications for TAVI over the past few years, such that this has now become the default therapy for a majority of patients with AS and aged 70 years or more.
The central features of an RCT are strict eligibility criteria, random allocation of participants to the strategies that are to be compared, the use of predefined measures of outcomes and effectiveness, and the inclusion of all randomised patients in the final analyses – resulting in a robust internal validity with few confounders. However, this also limits the applicability of RCT outcomes to daily clinical practice.
The landmark TAVI versus SAVR trials have typically only studied highly selected patients and have excluded AS patients with, e.g., bicuspid aortic valve, pre-existing aortic bioprosthesis, severe ventricular dysfunction, renal insufficiency and/or renal replacement therapy, and those patients assessed as being inoperable due to abdominal or thoracic aortic disease (e.g., porcelain aorta), hostile chest or a condition or complication from prior surgery that would preclude safe reoperation1,2,3,4,5,6. In addition, only patients suitable for safe transfemoral TAVI, with a low risk of coronary artery occlusion, and without severe AV calcification or calcification in the left ventricular outflow tract were included in the low-risk PARTNER 3 trial3. In this way, one third of patients screened for the PARTNER 3 trial were excluded based on anatomical criteria. Owing to these strict inclusion and exclusion criteria, this can limit the translatability of the results to routine clinical practice and result in a poor external validity and generalisability. Furthermore, decisions on study design, often influenced by the study sponsor, may indirectly impact on the study outcomes. In this context, the choice of the pre-specified primary endpoint in the PARTNER 3 trial – a composite of death, stroke, or rehospitalisation at one year – as well as the relatively high rate of concomitant cardiac surgery in the SAVR group could be criticised3.
Post-market studies and registries
Considering the limitations of RCTs, conducting real-world post-market studies and reporting registry data are important. Real-life studies have higher external validity than RCTs owing to less strict exclusion criteria and the ability to include a patient sample that is more representative of an all-comers patient population. All TAVI devices have their own post-market studies, e.g., SOURCE 3 for the SAPIEN 3 valve (Edwards Lifesciences, Irvine, CA, USA), FORWARD/FORWARD-PRO for Evolut™ R/PRO (Medtronic, Minneapolis, MN, USA), PORTICO I for Portico™ (St. Jude Medical [now Abbott Vascular], St. Paul, MN, USA), SAVI-TF for ACURATE neo™ (Boston Scientific, Marlborough, MA, USA), and RESPOND for Lotus™ (Boston Scientific). These real-world studies and registries can provide additional insights into areas such as epidemiology and typically aim for a five-year follow-up. These studies also provide the ability to generate hypotheses and study the device performance in “off-label use” scenarios, such as TAVI to treat bicuspid AS, degenerated aortic bioprostheses, elliptical or very small/large aortic annuli.
Real-world studies do have drawbacks that must be considered when assessing treatment benefits. Although many of these (especially industry-driven) post-market studies have an events committee, underreporting of clinical events may be a problem. This probably also partially explains the lower stroke and rehospitalisation rates reported in most TAVI registries as compared with the pivotal TAVI trials. As the patient population in registries is more heterogeneous, it can be difficult to control for confounding factors. Also, extrapolating registry findings, e.g., TAVI outcomes in the elderly with bicuspid AS, to a more general population may be tempting. However, an RCT will be needed to assess the true efficacy of TAVI versus SAVR in younger patients with bicuspid AS, as the underlying valve pathology and degree of calcification may be different between these populations.
Head-to-head THV comparisons
Following the completion of the pivotal TAVI trials in patients with high, intermediate and low surgical risk, an increasing number of head-to-head THV comparison studies can be expected. Although these studies are typically well conducted and strictly controlled, their outcomes should be assessed and interpreted with caution.
The REPRISE III trial was an industry-driven head-to-head study comparing two different TAVI platforms, the Lotus versus the CoreValve® platform (Medtronic), including both CoreValve and Evolut R8. The primary effectiveness endpoint (a composite of all death, disabling stroke and moderate or greater paravalvular leak [PVL] at one year) was significantly lower with the Lotus compared to the CoreValve platform (15.8 vs 26%, p<0.001). This outcome was mainly driven by a lower rate of moderate or greater PVL in those patients treated with the Lotus as compared to the CoreValve platform (0.9 vs 6.9%). However, the reason for and relevance of including moderate or greater PVL in the combined primary endpoint could be questioned, as the Lotus THV was a priori well known to have low PVL rates but, on the other hand, for example, also higher post-TAVI pacemaker implantation rates. Thus, a biased choice of a primary study endpoint may impact on the study outcome.
The PORTICO IDE trial highlighted another important aspect that may be considered when assessing comparisons between two TAVI platforms, namely the confounding effect introduced by the learning curve for the newer device. In the PORTICO IDE trial, patients were randomised between the Portico THV and two well-established FDA-approved commercial TAVR systems (65% SAPIEN, 35% CoreValve/Evolut). The reported safety and effectiveness for Portico improved from the first to the second half of the enrolled patients, underlining the importance of a learning curve when evaluating the outcomes of a newer device against those of more established THV platforms (Fontana GP; on behalf of PORTICO IDE Investigators. Primary outcomes of the PORTICO randomized IDE trial. Presented at TCT 2019, San Francisco, CA, USA, 27 September 2019).
Another recent head-to-head comparative TAVI trial was SCOPE I9, comparing the newer ACURATE neo with the more established SAPIEN 3 TAVI system. ACURATE neo did not meet non-inferiority compared to the SAPIEN 3 in terms of early safety and clinical efficacy outcomes due to higher rates of PVL and acute kidney injury. Also, for the newer ACURATE neo device, a learning curve effect should be taken into consideration. Moreover, although all THV platforms may be used in a wide clinical spectrum, experienced TAVI operators are aware of the fact that there are some specific indications and anatomies where a specific device is particularly suitable or, on the other hand, less suitable. For the ACURATE neo, one feature is a lower radial force, which results in a lower risk of damage to the conduction system, but which can also be a limitation in severely calcified aortic valves (and hence result in an increased risk for PVL in such cases). On the other hand, the ACURATE neo has a supra-annular leaflet position, which is associated with better valve haemodynamics and less patient-prosthesis mismatch compared to THVs with an intra-annular leaflet position. The lack of such understanding may influence procedural and study outcomes. A more patient-tailored TAVI approach is the reality in daily clinical practice, but it collides with the blind (non-tailored) 1:1 randomisation in these head-to-head comparative studies.
Finally, with the longer life expectancy of the currently treated younger patients undergoing TAVI, the prosthetic valve choice will become increasingly important, as aspects other than early device safety and efficacy will gain importance, such as the long-term effects of conduction abnormalities, access to the coronary arteries, patient-prosthesis mismatch, valve durability, etc. Comparative studies investigating the impact of THV choice on these issues will demand longer-term follow-up than the current head-to-head comparative TAVI studies.
Conclusion
In conclusion, RCTs are performed with the primary goal of understanding the efficacy of a new therapy in a selected group of patients. Even when evidence from well-designed and well-conducted RCTs strongly favours a new therapy, real-world studies and registries are needed as these provide a “bridge” to daily clinical practice. Both RCTs and registries are complementary, and their respective advantages and disadvantages must be considered when designing the studies, analysing the data, and interpreting the study outcomes.
Conflict of interest statement
O. De Backer has received institutional research grants from and has been consultant for Abbott and Boston Scientific. L. Søndergaard has received consultant fees and institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic and Symetis.

