Abstract
Cumulative evidence has demonstrated that transcatheter aortic valve implantation (TAVI) constitutes an effective treatment option for patients with severe symptomatic aortic stenosis and a high operative risk. New valve designs and TAVI-enabling devices have simplified the procedure, reduced the risk of complications, and broadened the applications of this treatment. The global adoption of TAVI allows us to appreciate the advantages, potentialities and caveats of the technology, identify patients who would benefit from TAVI and stratify more accurately the risk of complications. The focus of this article is to discuss the advances in this field, present the current evidence, and highlight the developments and strategies proposed to address the limitations of TAVI treatment.
Introduction
Transcatheter aortic valve implantation (TAVI) was introduced over a decade ago by Cribier and colleagues and has revolutionised the treatment of symptomatic severe aortic stenosis (AS). Its less invasive nature, compared to surgical aortic valve replacement (SAVR), appeared particularly attractive for the treatment of high-risk or inoperable patient cohorts. Multiple prospective registries and the randomised Placement of AoRTic TraNscathetER Valves (PARTNER) trial support the broad application of this treatment in patients with a (very) high operative risk1.
The global adoption of TAVI allowed us to appreciate its potential, refine patient selection and optimise device and treatment strategies so as to reduce the risk of complications. In recent years several trials have been organised to explore the efficacy of TAVI in different populations and a considerable effort has been made towards the development of new more effective valves and TAVI-enabling devices that would simplify the procedure, increase safety, and permit treatment of patients with a complex valve anatomy. In this review article we present the current evidence stemming from registries and clinical trials, cite the upcoming or ongoing clinical studies, discuss the limitations of TAVI treatment and provide an update about the recent advances in the field.
Clinical evidence
Three randomised controlled trials have examined the efficacy of TAVI in different populations. The PARTNER Cohort B trial demonstrated a 20% absolute reduction in all-cause mortality at one-year follow-up and a better quality of life in inoperable patients undergoing TAVI as compared to those receiving optimal medical therapy, including balloon valvuloplasty1. The PARTNER Cohort A trial randomised patients at high operative risk to TAVI or SAVR and showed no difference in mortality between the two groups at two-year follow-up. TAVI patients experienced more neurological events or major vascular complications whereas SAVR patients had more bleeding complications2. The STACCATO trial randomised elderly low-risk patients (>75 years old) to transapical TAVI or SAVR. The study was prematurely ended after enrolling only 70 patients as there was an increased event rate in the TAVI arm3.
Results from large national registries such as the United Kingdom, the French, and the German registries confirmed the findings of the randomised trials, underscored a high success rate and an acceptable periprocedural event rate, and provided additional data about the short and mid-term efficacy of TAVI treatment4-6. Although there is robust evidence to support the short- and mid-term effectiveness of TAVI, there is limited data about the long-term efficacy of the implanted devices. The first report that assessed the long-term performance of TAVI, included 70 patients implanted with an Edwards SAPIEN THV prosthesis (Edwards Lifesciences, Irvine, CA, USA) who were followed-up for 3.5 years and had serial echocardiographic evaluation and computed tomographic (CT) imaging at three-year follow-up7. Only one patient had a re-operation because of endocarditis. Echocardiography demonstrated stable prosthesis function with only a minor decrease in the aortic valve area (by 0.06 cm2/year, p<0.001) and no deterioration in the reported aortic regurgitation, while CT did not show evidence of leaflet thickening, fusion or calcification nor frame fracture. Similar were the results of the Italian CoreValve registry which showed no significant changes in the transaortic valve gradient or the reported aortic insufficiency at three-year follow-up8. Recently Rodes-Cabau provided further insights into valve durability presenting the five-year follow-up data of the Canadian Multicenter Experience study9. A total of 333 patients underwent TAVI with an Edwards SAPIEN THV device. Serial echocardiography demonstrated a gradual decrease in the aortic valve area at two-year follow-up (from 1.62±0.38 cm2 to 1.44±0.33 cm2 at one year, and 1.38±0.35 cm2 at two years, p<0.007) which did not change at three-year (1.36±0.38 cm2) and four-year follow-up (1.35±0.37 cm2). These findings however, require further confirmation as in this study only 50 patients had echocardiographic evaluation at 3-year, and 28 patients at 4-year follow-up.
The Medtronic CoreValve® U.S. Pivotal Trial (NCT01240902) randomising AS patients at high operative risk to TAVI with the Medtronic CoreValve® (Medtronic, Minneapolis, MN, USA) or SAVR and also exploring TAVI with the Medtronic CoreValve in inoperable patients (not randomised) has recently completed enrolment and the results are anticipated in due course. Two randomised clinical trials are exploring TAVI vs. SAVR in patients at intermediate operative risk: the PARTNER II Cohort A (ClinicalTrials.gov, number NCT01314313) and the SURgical and Transcatheter Aortic Valve Implantation (SURTAVI) trial (ClinicalTrials.gov, number NCT01586910). PARTNER II Cohort A will randomise about 2,000 patients and SURTAVI 2,500. Both are non-inferiority studies and will include patients with coronary artery disease amenable by percutaneous coronary intervention (Figure 1). Finally, the PercutAneous Coronary inTerventIon prior to transcatheter aortic VAlve implantaTION (ACTIVATION) trial has recently commenced and aims to randomise 310 patients with ischaemic coronary artery disease to direct TAVI and percutaneous coronary intervention before TAVI on a 1:1 basis so as to examine the effect of revascularisation on clinical outcomes at one-year follow-up.
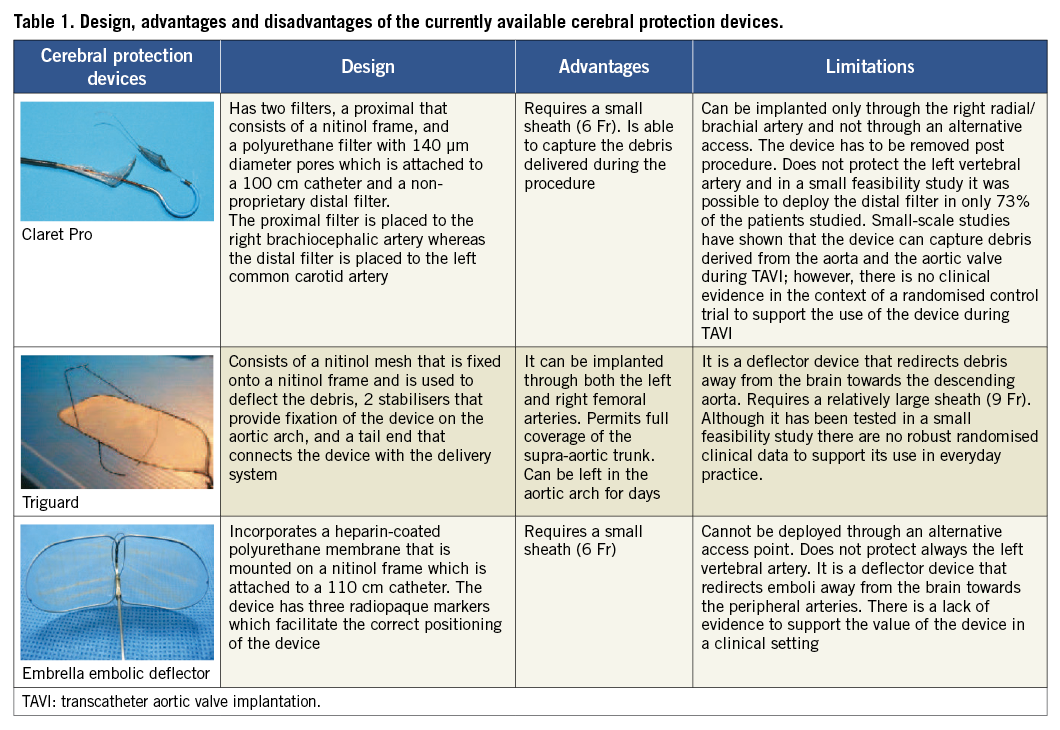
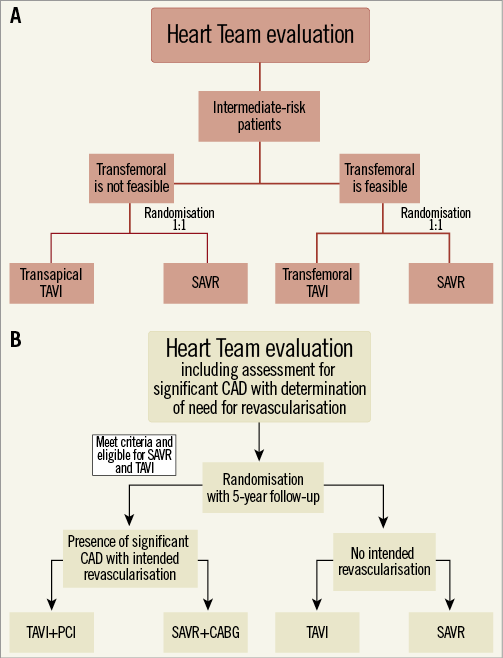
Figure 1. Flow chart of the PARTNER II Cohort A (A) and the SURTAVI trials (B). TAVI: transcatheter aortic valve implantation; CAD: coronary artery disease; PCI: percutaneous coronary intervention; SAVR: surgical aortic valve replacement
Complications in TAVI
Stroke, vascular complications, a dysfunctional prosthesis (i.e., device malapposition and migration or paravalvular leak), and conduction abnormalities are the most frequent complications associated with TAVI, whereas aortic dissection and rupture, coronary ostium obstruction, periaortic haematoma, tamponade and mitral injury are rare but potentially fatal acute complications. The definitions of these complications have recently been standardised by the Valve Academic Research Consortium-2 consensus document10. New valve designs and TAVI-enabling devices have been developed to simplify the procedure, reduce the risk of adverse events and allow the use of this treatment in complex anatomies and a broader number of patients.
CEREBROVASCULAR EVENTS
Cerebrovascular events (CVE) negatively impact survival and quality of life in patients treated with TAVI. Recent studies have provided additional evidence about the incidence and timing of these events and showed that most of the CVE occur within the first 24 hours post procedure and that their incidence remains high for the first two months after device implantation. Multiple manipulations during implantation such as repositioning and post-dilation of the prosthesis appear to augment the risk for CVE11.
Magnetic resonance imaging and transcranial Doppler studies have shown that TAVI is associated with new cerebral lesions, supporting the hypothesis that most of the CVE have embolic aetiology; while recently van Mieghem et al investigated the histopathology of the embolised material and demonstrated that most of the debris that travels to the brain derives either from the native aortic valve or the aortic wall12,13. Brain hypoperfusion during device deployment has also been considered as a potential cause of stroke, whereas new onset atrial fibrillation is associated with the CVE occurring between the first 24 hours and the first two months post-procedure14.
To reduce the risk of CVE, three cerebral embolic protection devices have been developed which were tested in small clinical studies (Table 1)13,15,16. Direct implantation of the valve without balloon predilation is also likely to decrease the risk of CVE. The SIMPLIFy TAVI trial has recently commenced and aims to randomise 110 patients with severe AS and an ejection fraction <35% to TAVI with and without predilation and compare the 30-day event rate (all-cause mortality, stroke, non-fatal myocardial infarction, acute kidney injury, and pacemaker implantation) in the two groups (ClinicalTrials.gov, number NCT01539746).
Antiplatelet treatment is strongly recommended for patients undergoing TAVI but there is a lack of evidence about the optimal regimen and the duration of treatment17. According to the recently published ACCF/AATS/SCAI/STS guidelines dual antiplatelet treatment with aspirin and clopidogrel should be initiated in all the patients, but the dose and the duration of the treatment has not been specified. Of note, a small-scale randomised trial that included 90 patients undergoing TAVI showed no difference in the prognosis between patients treated with dual antiplatelet treatment and those receiving only aspirin18. Further evidence is expected from the Aspirin versus aspirin and clopidogRel following Transcatheter Aortic Valve Implantation (ARTE) trial that is currently underway and aims to randomise 200 patients to aspirin versus aspirin and clopidogrel in order to compare the efficacy of these therapies in the prevention of major ischaemic events (ClinicalTrials.gov, number NCT01559298).
VASCULAR COMPLICATIONS
Vascular complications and major bleeding are significant limitations of TAVI. Their incidence is increased when TAVI is performed through the transfemoral route, which nonetheless constitutes the preferred access owing to its less invasive nature. Evaluation of the iliofemoral anatomy with multislice computed tomography (MSCT) and, more specifically, assessment of the vessel size, degree of calcification, plaque burden and tortuosity, allow identification of the patients who are suitable for transfemoral TAVI and reduce the risk of vascular complications. In a recent study, Van Mieghen et al examined the incidence, predictors, and implications of access-site complications in almost 1,000 patients implanted through the transfemoral approach19. They found that only the size of the sheath was a predictor of vascular complications, while major bleeding was independently related to female gender, larger sheaths, a history of peripheral vascular disease and percutaneous access and closure. Vascular complications and major bleeding were associated with worse outcomes.
Appreciating the effect of a sheath’s dimensions on vascular adverse events, an effort has been made in recent years to reduce the size of the delivery systems and improve the crossing profile of the new-generation valves. Edwards Lifesciences has recently introduced a low profile (16 Fr) expandable sheath called eSheath that expands transiently when it is in the aorta to allow the passage of the mounted valve and then returns to its natural dimensions. Terumo (Terumo Corporation, Somerset, NJ, USA) has also developed a less traumatic sheath SoloPath® with an even lower crossing profile (diameter of the distal tip of 13 Fr) that can be used for the deployment of the Medtronic CoreValve. SoloPath is also expanded in the aorta with the use of a balloon to allow passage and deployment of the valve. The performance of this low-profile sheath was recently tested in a small feasibility study that included 27 patients with a complex, borderline iliofemoral anatomy. The vascular complications rate in this high-risk population was 18% which is comparable to the event rate reported in studies conducted in unselected patients, highlighting the potential value of this device in the management of patients with an unfavourable iliofemoral anatomy20.
The smaller size of the new sheaths has permitted the development of closure devices for a controlled and safe sealing of the access site. To date, three devices have been tested in a clinical setting: the Prostar® (Abbott Vascular, Santa Clara, CA, USA) and the ProGlide (Abbott Vascular, Santa Clara, CA, USA) for the transfemoral and the APICA ASC (APICA Cardiovascular, Galway, Ireland) for the transapical approach, while others such as the ProMed (Promed, Santa Clara, CA, USA) and the InSeal (InSeal Medical, Caesarea, Israel), for the transfemoral access and the Permaseal™ (Micro Intervention Devices, Bethlehem, PA, USA) for the transapical route are currently undergoing first-in-man studies21. Finally, two devices, the VasoStitch (VasoStitch Inc., Danville, CA, USA) for the transfemoral and the CardioClose (Entourage Medical, Menlo Park, CA, USA) for the transapical approach, are under preclinical evaluation.
PROSTHESIS DYSFUNCTION
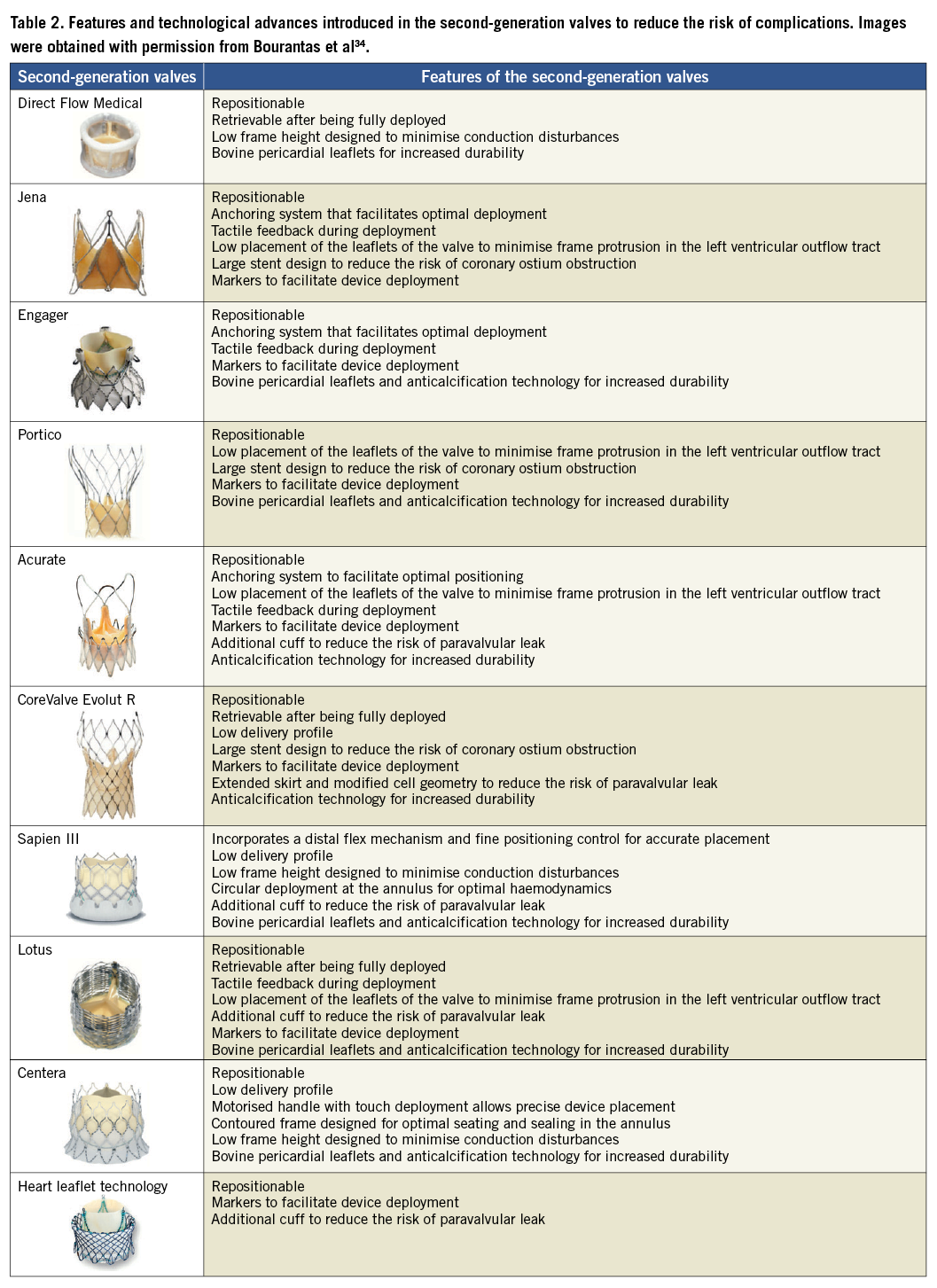
Suboptimal valve positioning is a common problem seen in TAVI. The first-generation valves, i.e., the Edwards SAPIEN THV, the Edwards SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA) and the CoreValve® could not be retrieved after positioning and thus, in case of an erroneous placement, the operator had to deploy a second device or refer the patient for urgent SAVR. To overcome this limitation several new valve systems have been developed that can be repositioned or even be retrieved if needed. Some of them have been tested in a clinical setting and a few have already acquired Conformité Européenne (CE) mark approval such as: the Direct Flow Medical valve (Direct Flow Medical Inc., Santa Rosa, CA, USA) that is both retrievable and repositionable, the JenaValve (JenaValve, Munich, Germany) and the Portico (St. Jude Medical, St Paul, MN, USA) that are both retrievable until being fully deployed, the Engager that is only repositionable (Medtronic, Minneapolis, MN, USA) , and the Symetis® Acurate™ (Symetis SA, Ecublens, Switzerland) that has a self-seating and self-sealing design that conforms with the native anatomy potentially leading to a lower incidence of paravalvular leak (Table 2).
Paravalvular aortic regurgitation after TAVI has been attributed to suboptimal device sizing and positioning. Two recently published studies have provided convincing evidence about the prognostic implications of a significant (≥+2/4) aortic regurgitation22-23. Accurate evaluation of valve anatomy and sizing of the prosthesis appear to reduce the risk of paravalvular regurgitation. Today, several imaging modalities are available for this purpose including 2-dimensional (2-D), 3-D and transoesophageal echocardiography, MSCT, x-ray aortography and magnetic resonance imaging. These modalities also allow assessment of the distance between aortic valve annulus and left main stem ostium, which is important for a successful procedure (i.e., a low origin of the left main stem has been associated with an increased risk of left main obstruction), evaluation of the calcification in the leaflets and annulus that can affect treatment planning, and assessment of the take-off of the ascending aorta (i.e., horizontal or vertical take-off) which is important as a horizontal take-off may hinder correct and coaxial device deployment (Figure 2)24.
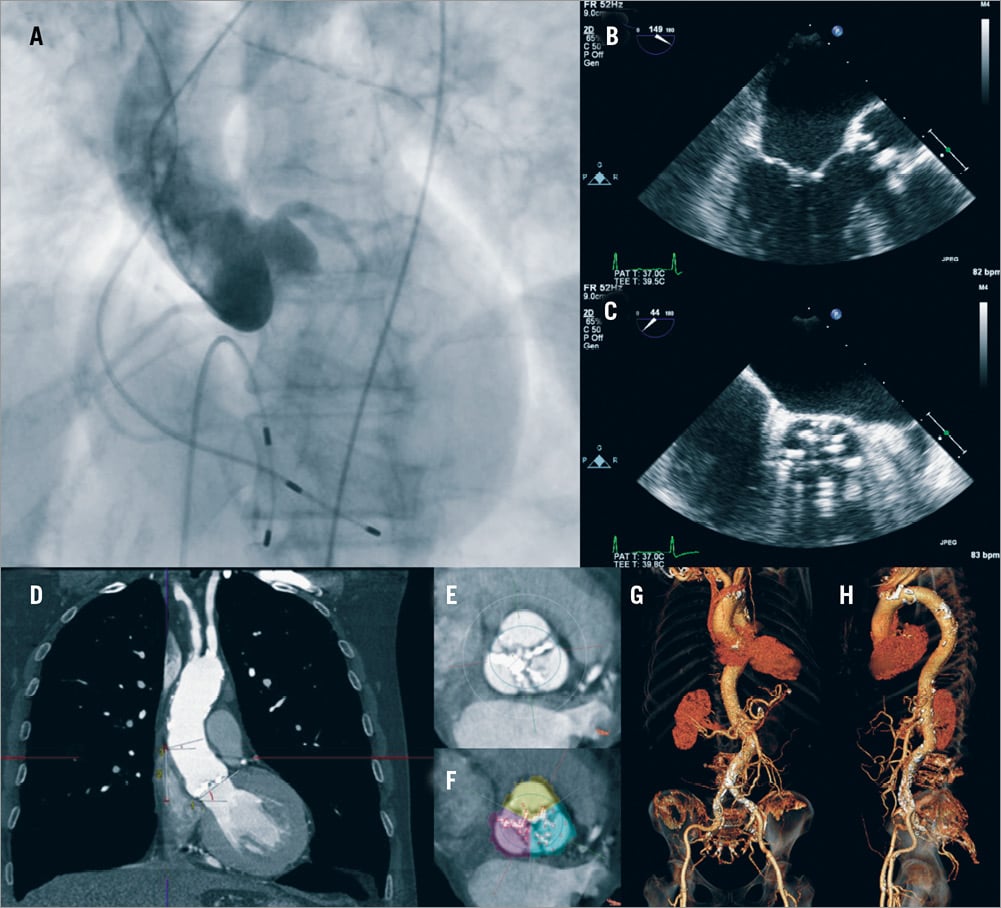
Figure 2. Imaging modalities used in the SURTAVI study to assess patients’ suitability and procedural planning. (A) x-ray angiography is used to assess coronary anatomy and the morphology of the aorta; (B, C) transoesophageal echocardiography allows evaluation of aortic valve anatomy measurement of its dimensions and quantification of the severity of the aortic stenosis; (D-F) multislice computed tomography (MSCT) provides comprehensive images that permit accurate evaluation of the take-off of the aorta, and of the ostium of the left main stem and right coronary artery, assessment of the dimensions of the aortic root and valve and quantification of the calcification of the valve; (G-H) in addition MSCT provides detailed visualisation of the iliofemoral arteries and of the descending aorta, assessment of vessels’ tortuosity and measurement of their dimensions.
Recent advances in imaging and image processing technology have allowed the development of new systems and data fusion techniques that permit comprehensive, real-time visualisation of the aortic valve anatomy and can be used in the catheterisation laboratory to guide treatment. The C-THV Paieon (Paieon Inc, New York, NY, USA), the 3mensio valve™ system (3mensio Medical Imaging, Bilthoven, NL), the Phillips heart navigator (Phillips, Best, NL), and the DynaCT (Siemens AG, Erlangen, Germany) are software that can process angiographic or MSCT data to reconstruct coronary anatomy and define the best angiographic projection for treatment planning. Recent proof-of-concept studies validating these systems demonstrated a reduction in the paravalvular regurgitation in patients treated with the use of these software25,26.
CONDUCTION DISTURBANCES
Conduction abnormalities are common complications seen during TAVI, especially in patients implanted with the self-expanding systems that are deployed deeper in the left ventricular outflow tract and exert a long-lasting radial force that can injure the conduction system of the heart. Although there is evidence that conduction abnormalities, which often require pacemaker implantation, may affect left ventricular systolic function and functional status, their effect on prognosis is questionable with most of the recently published studies showing no association between conduction abnormalities and prognosis and one study demonstrating increased mortality in the patients who develop left bundle branch block during TAVI27-29.
To reduce the risk of conduction abnormalities the second-generation self-expanding valves have a low frame height or a low placement of their leaflets in their frame that permit a minimal protrusion of the valves in the left ventricular outflow tract.
New developments in TAVI technology
Apart from the devices mentioned above that have already acquired CE mark approval, several other valves have been designed that are currently undergoing clinical evaluation such as: the Sapien III (Edwards Lifesciences, Irvine, CA, USA) which incorporates an additional cuff that covers the frame of the valve and is anticipated to reduce the incidence of paravalvular leak; the Lotus valve that is both retrievable and repositionable (Boston Scientific, Natick, MA, USA), the CoreValve Evolut R (Medtronic, Minneapolis, MN, USA) that has a low delivery profile; the self-expanding Centera (Edwards Lifesciences, Irvine, CA, USA) valve which has a low frame height to reduce the risk of conduction disturbances; as well as the HLT™ (HLT Inc., Maple Grove, MN, USA) valve that was initially implanted in humans in 2009 where it was found to be associated with an increased risk of complications. This last device has recently been redesigned and currently there is a plan for clinical evaluation (Table 2).
To reduce the risk of pericardial effusion, or ventricular perforation, caused by the stiff guidewires used in TAVI procedures Roy et al have introduced a dedicated TAVI guidewire30. The proposed device is made of stainless steel that tapers gradually towards its tip, which is covered by polytetrafluoroethylene, and has a pre-shaped curve that performs at least one revolution. This design is anticipated to reduce the local force that the other wires often exert to the left ventricle during prosthesis implantation. The proposed guidewire was evaluated in a small study that included 39 patients. In all cases the guidewire was successfully positioned into the left ventricle and none of the patients experienced guidewire-related procedural complications.
Future trends in TAVI
Several small-scale studies have provided evidence that TAVI has a value for the treatment of complex patients with a failed surgically implanted bioprosthesis31. It is unclear what sizing, positioning and deployment principles should be followed during device implantation but it seems that valve-in-valve TAVI is gradually establishing itself as a valid alternative.
A bicuspid aortic valve anatomy and a dysfunctional aortic valve with severe aortic regurgitation were considered as contraindications for TAVI. However, recent small-scale studies have shown that TAVI may be an effective alternative for the treatment of severe symptomatic aortic regurgitation or AS in patients with a bicuspid valve32-33. Although the small number of patients included in these studies does not allow us to draw firm conclusions, it is likely that in the future the applications of TAVI will expand.
Conclusions
TAVI has changed the landscape of interventional cardiology, introducing an attractive, less invasive option for the treatment of symptomatic AS. Growing evidence from large registries and randomised controlled trials has underscored the efficacy of TAVI and supported its use in selected populations, while the technological advances in valve design and TAVI-enabling devices have facilitated the procedure, reduced the risk of complications and broadened the applications of this treatment. Although there has been a tremendous evolution in this field in recent years, further research is required to confirm the long-term efficacy of the implanted devices, identify the patients who will benefit from this treatment, stratify more accurately the risk of complications, minimise periprocedural adverse events and optimise post-procedural management.
Funding
C.V. Bourantas is funded by the Hellenic Cardiological Society, Athens, Greece.
Conflict of interest statement
The authors have no conflicts of interest to declare.

