Introduction
Transcatheter aortic valve implantation (TAVI) is a life-prolonging treatment in patients deemed ineligible for surgical aortic valve replacement1. Non-invasive imaging is essential for evaluating patient eligibility, planning of vascular access and sizing, which is selecting a prosthesis size that matches the patient anatomy. A joined ESC/EACTS/EAPCI committee recommended patient selection based on multiple imaging modalities2.
In the majority of TAVI cases performed so far, the preprocedural planning has been done based on two-dimensional (2D) imaging modalities including echocardiography and contrast angiography (CA). However, it is now recognised that the aortic root has a complex three-dimensional (3D) anatomy and that the aortic annulus, on which sizing is based, is non-circular3. Recent reports show that anatomical factors may affect procedural outcome, including the incidence of paravalvular aortic regurgitation, the need for balloon post-dilatation and pacemaker requirements4-6.
Three-dimensional modalities such as multislice computed tomography (MSCT) have advantages for imaging the complex anatomy of the aortic root and allow evaluation of all potential vascular access routes2,3,7.
Conventional evaluation of MSCT data requires both a 3D workstation as well as expertise that are seldom available outside of the radiology department.
History
Patient Archiving Communications Systems (PACS) allow the viewing of only axial MSCT slices that usually preclude accurate measurements of 3D structures. Free 3D Dicom viewers are available but are complex and time-consuming to learn to use.
Advanced 3D workstations are versatile to enable the evaluation of all aspects of human anatomy but are consequently complex, expensive and time consuming to learn. Logistics may also limit access.
3mensio Valves™ was developed to enable cardiologists and cardiothoracic surgeons to plan TAVI procedures by directly using MSCT volumes, with minimal user interaction and a simple learning curve.
Description
The 3mensio Valves™ package has workflows focused specifically on TAVI procedures and provides multimodality and advanced 3D capabilities. The workflows include analysis of the aortic root (valve analysis), transfemoral, trans-subclavian and transapical access as well as echocardiography and CA.
All the MSCT workflows start with automatic segmentation of the structures of interest. The longitudinal and short axis views that are generated around a centreline can be interrogated from any angle. If necessary the centreline may be rapidly corrected by the user by dragging control points with the mouse. In cases of suboptimal image quality each workflow has an assisted manual option that gives full control over the centreline definition.
Figure 1 shows the first step of valve analysis. In this example the aortic root was found automatically after the user selected the dataset. After user confirmation, the view switches to Figure 2 which allows measurement of all anatomical patient eligibility parameters listed in the industry guidelines (as shown in Figure 2) including calcification and aortic leaflet morphology (Figure 3).
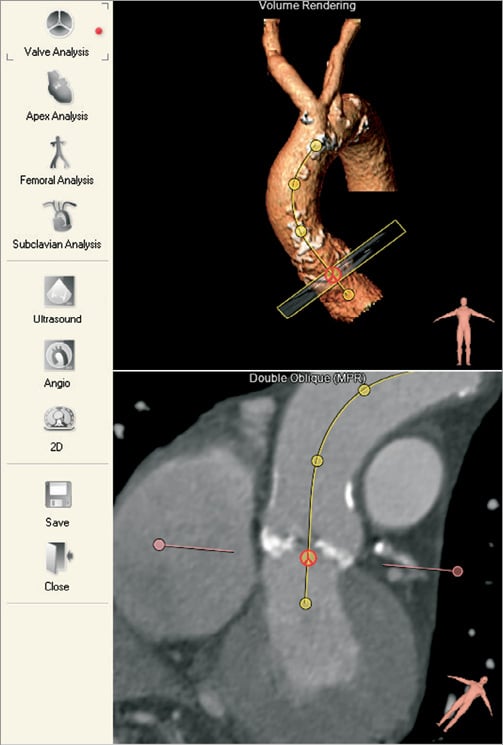
Figure 1. Automatic segmentation. The required workflow type is selected on icons (left). The valve analysis workflow automatically segments the aortic root (top right). The user confirms the segmentation and can adjust the basal plane (bottom right).
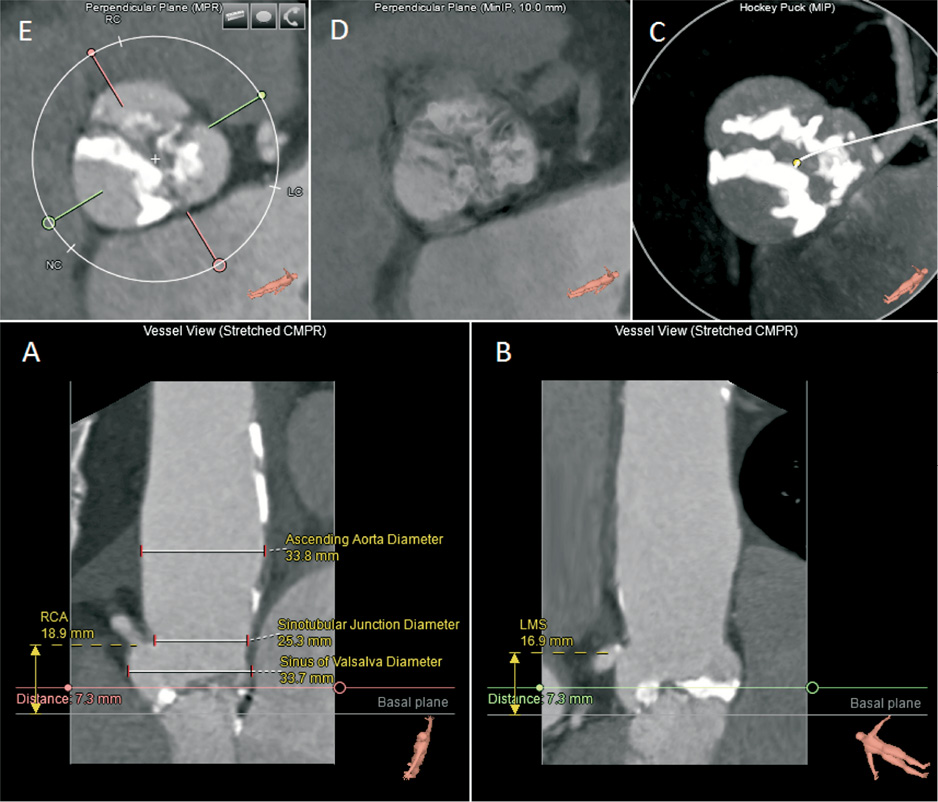
Figure 2. The valves workflow. The analysis screen shows straightened longitudinal views (A, B), which allow measurement of height and depth relative to the basal plane. View A shows the heights of the right coronary artery (RCA) from basal plane, the ascending aorta diameter, the sinotubular junction diameter and the sinus of valsalva diameter. View B shows the left main stem (LMS) height from the basal plane.Additional views include a short-axis “hockey puck” volume that enhances calcifications (C), a short-axis MinIP image that highlights the non-calcified (low-intensity) leaflets (D), and a standard short-axis view for measurements of axial diameters, area and perimeter (E).
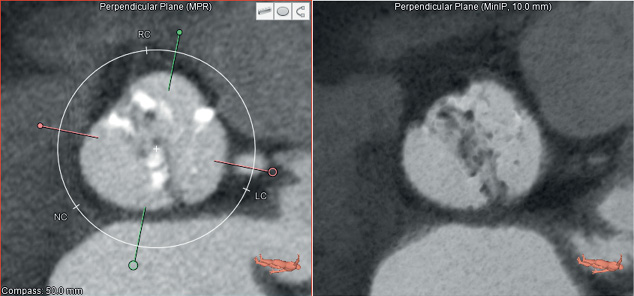
Figure 3. A short axis view of a bicuspid aortic valve. The standard (MPR) image is shown (left). The MinIP view (right) highlights low contrast (non-calcified) structures and in this example makes it easier to appreciate the bicuspid anatomy of the leaflets.
Thereafter, the C-arm angulation for the optimal angiographic view can be defined (Figure 4)3,8. The short-axis view allows the minimum and maximum diameters of the non-circular aortic annulus to be measured.
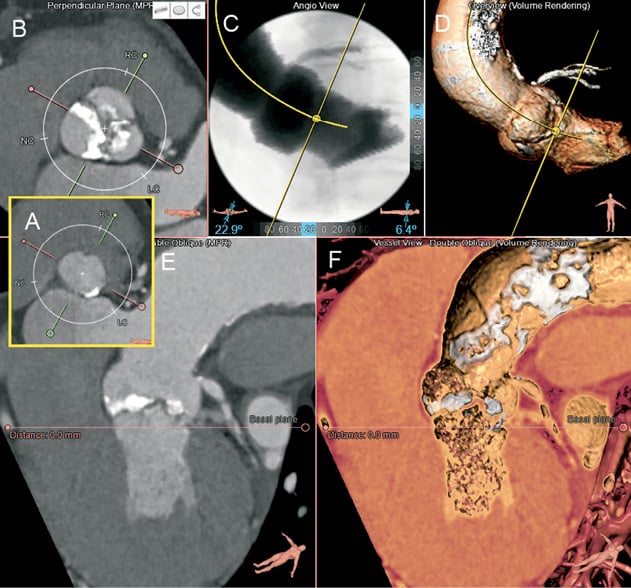
Figure 4. Planning the optimal C-arm angulation for the TAVI procedure. After locating the nadirs of the leaflets (A) the short-axis image is scrolled to the level of the sinuses (B). On the short-axis image the green line is rotated to cut through the centre of the right coronary sinus and along the coaptation line of the non-and left coronary leaflets (B) so that the orthogonal view will be given by the red line. The viewing angles (LAO 23, cranial 6) obtained can be reproduced on the angiography simulation view (C) and the 3D volume view (D) to verify that the bases of the three coronary sinuses are on one line with the right coronary sinus in the centre. E and F show a modified coronal and 3D views revealing significant aortic calcification.
The transfemoral and subclavian workflows allow evaluation of potential arterial catheter access routes as shown in Figure 6 and Figure 7.
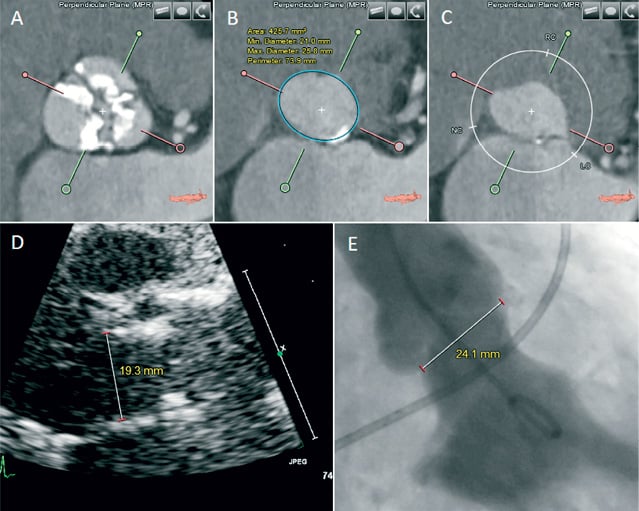
Figure 5. Measurement of aortic annulus dimensions. On the short-axis view, the basal plane is set at the nadir of the three aortic leaflets (B). This enables axial measurements of the noncircular aortic annulus (e.g., minimum and maximum diameter, area and perimeter) and also at the level of the sinuses (A) and the left ventricular outflow tract (C). For the aortic annulus the corresponding measurements on transthoracic echocardiography (D) and contrast ventriculography (E) are shown.
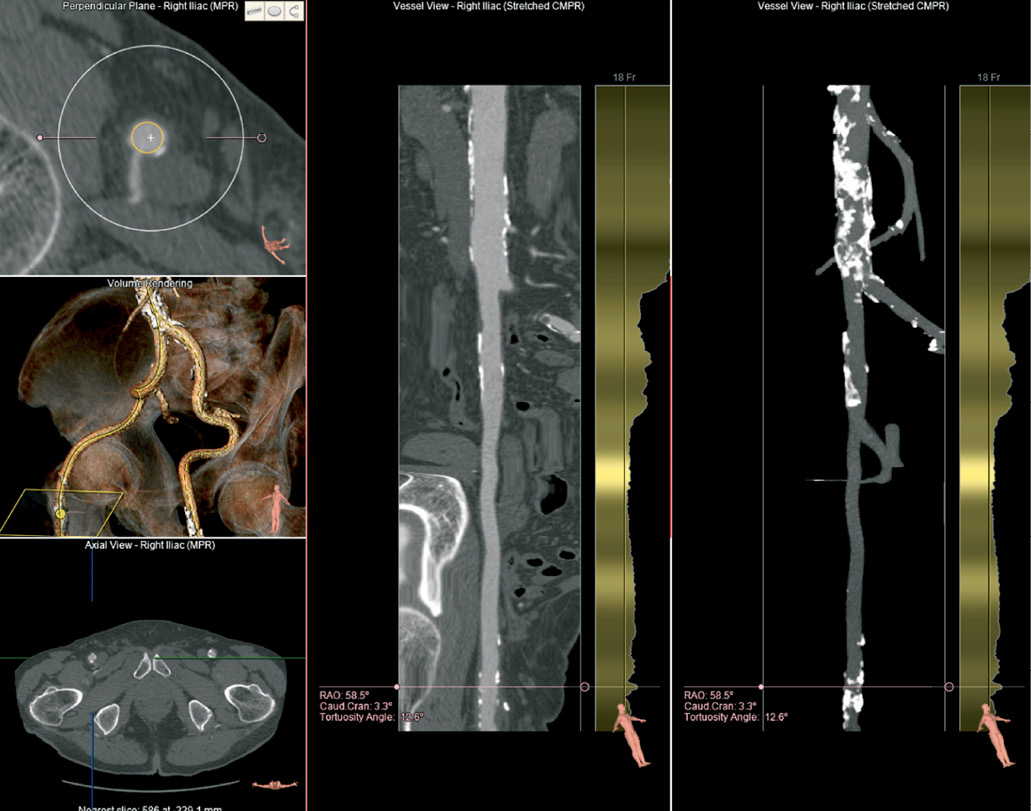
Figure 6. Evaluation of trans-femoral access. A 3D volume image (left middle) allows an overview of tortuosity and calcification and localisation of the short-axis plane (left top). A straightened view of the right femoro-iliac vessel (middle panel) can be rotated along the central axis. An overview of the calcifications is shown on the right panel. Automated lumen diameter detection is shown to the right of the stretched views with reference lines that indicate the delivery catheter diameter.
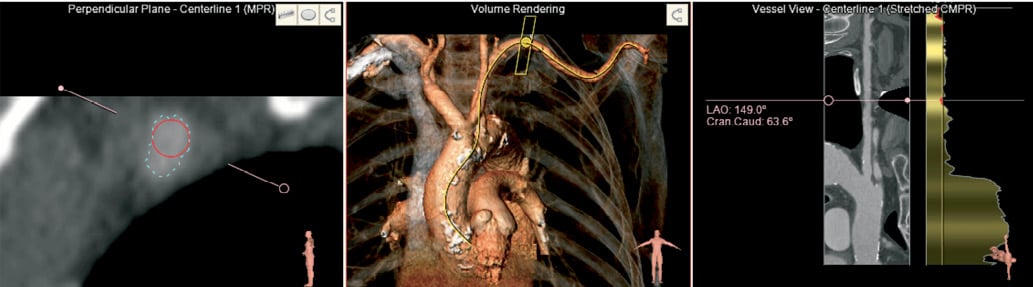
Figure 7. Evaluation of trans-subclavian access. The 3D volume (middle panel) shows the location of the short axis view (left) where the lumen perimeter (blue) and the minimal diameter (red) is seen. A stretched vessel view shows the minimum diameter along the centre line.
Transapical analysis is simpler as it does not require segmentation as shown in Figure 8. The path of access through the ribcage and the angle with the aortic annulus can be evaluated. In complex cases where all transvascular and transapical access prove to be not feasible this flexible tool can also be used to evaluate alternative approaches, for example, direct aortic access.
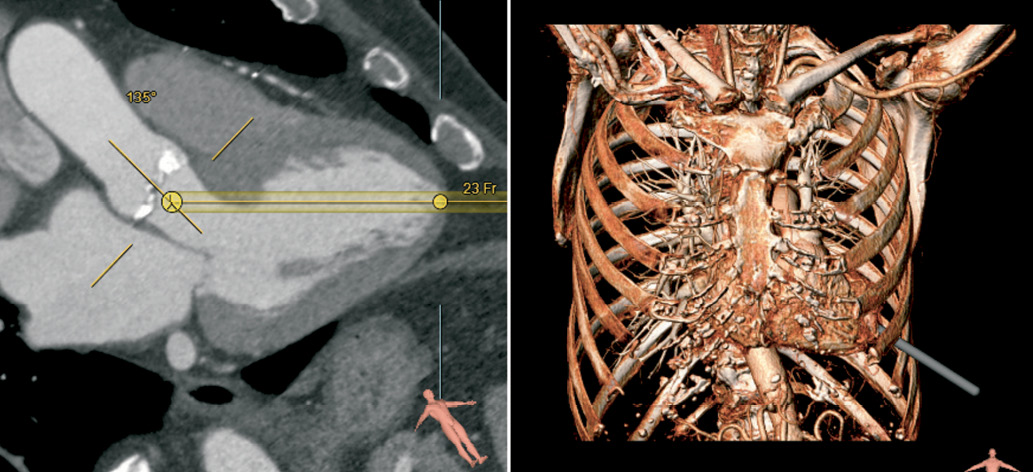
Figure 8. Evaluation of the transapical catheter path. The user selects the position of the left ventricular apex and the aortic leaflets. Subsequently the view can be rotated around the virtual catheter (left panel). On the 3D volume the simulated transapical catheter path, suggests catheter entry between ribs 6 and 7.
Separate workflows are provided to analyse CA and echocardiographic images. All conventional measurements including distances, angles and Doppler gradients are available. All measurements from all workflows and all modalities can be centralised in one comprehensive report, along with corresponding images.
Technical details
The 3mensio software can be loaded on modern desktop computers and notebooks that have 3D hardware acceleration.
Data sets have to be in DICOM format. For optimal image quality ECG-gated, contrast enhanced MSCT data sets are required for the aortic root analysis. To evaluate the access routes, a single contrast enhanced data set ranging from the subclavia to the femoral bifurcation may be used or individual data sets for the thoracic and abdominal vasculature. The data can be loaded from a PACS server, a DICOM CD or USB drive.
Indications
The software is indicated for:
– evaluation of eligibility for TAVI
– planning of TAVI access route (transfemoral, transapical, subclavian)
– selection of optimal angiographic view
– prosthesis size selection
Tips and tricks for use
All modules work with approximately the same steps, making it easy to navigate other modules after learning the first.
All measurements can be performed via the mouse.
Measurement results and screenshots can be integrated into a single report including all workflows and imaging modalities.
Clinical experience
The system has been compared to conventional MSCT software for analysis of the aortic root and was found to have a higher reproducibility of measurement. The semi-automated analysis approach also reduced analysis time9. In a clinical study where a series of 110 patients were evaluated using 3mensio software, it was reported that calcification of the aortic root and annulus diameter were predictive of the need for balloon post-dilatation in order to reduce a paravalvular aortic regurgitation during the TAVI procedure10.
Conflict of interest statement
J. de Vaan and L. Verstraeten are employees of 3mensio Medical Imaging BV. The other authors have no conflict of interest relevant to the subject matter of this publication.