Transcatheter aortic valve implantation (TAVI) is a valid treatment option for patients with symptomatic aortic stenosis (AS) and a high operative risk1,2. Transfemoral arterial access is generally considered the access site of first choice and is feasible in a completely percutaneous fashion. In case of unfavourable femoral-iliac arterial vessels, the transapical, transaxillary (or trans-subclavian) and direct aortic access are alternative strategies for TAVI, yet require surgical techniques3. We present the first transaxillary TAVI using an exclusively percutaneous approach and focus on the step-by-step key aspects of this strategy.
A 78-year-old male was accepted for TAVI because of severe symptomatic aortic valve stenosis and extensive past medical and surgical history. By multislice computed tomography (MSCT) scan, we observed that the femoral-iliac arterial vessels were diffusely diseased and considered incompatible with large sheath cannulation; the subclavian and axillary arteries had a minimal luminal diameter of 1 cm, the left subclavian artery demonstrated an indentation at the crossing of the first rib (Figure 1) and the left internal mammary artery (LIMA) was grafted to the LAD. A right transaxillary arterial route was therefore selected using a completely percutaneous technique. After in-depth internal discussion among the “valve team” consisting of interventional cardiologists, cardiothoracic surgeons, imaging specialists and anaesthesiologists, a consensus for a completely percutaneous approach was reached because the axillary artery was compressible to a certain extent and the surgeon was scrubbed in and ready to perform a surgical cut down in the case of unforeseen complications that would not be manageable with percutaneous techniques.
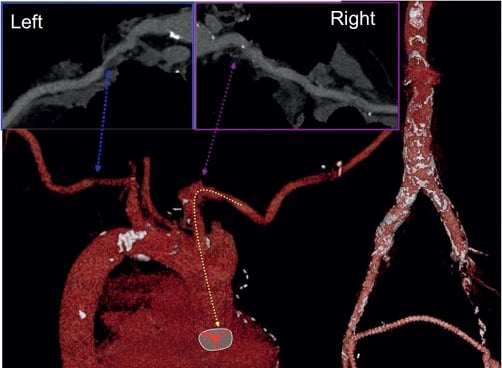
Figure 1. Multislice computed tomography scan illustrating the right axillary/subclavian artery course.
The following anatomical principles were taken into account with respect to the percutaneous axillary arterial access. Along the median portion of the clavicle, the subclavian vein runs above and in front of its arterial counterpart. More medially the subclavian artery briefly courses above the clavicle and then posteriorly and centrally towards the mediastinum. Lateral to the first rib, the vascular structures segregate and the subclavian artery extends into the axillary artery, partly released from overlying major venous structures (Figure 2). From a procedural perspective with a percutaneous approach in mind, a supraclavicular approach with puncture of the subclavian artery was deemed less favourable because of the relative vicinity of the brachial plexus and the potential need for bailout major surgery including claviculotomy in case of significant bleeding and vascular complications. Ultrasound examination would allow for precise location of the axillary artery without anterior subclavian/axillary vein interference and could help in real time ultrasound guided puncture4. When engaging the axillary artery with the 18 Fr sheath, it was essential not to perforate any overlying venous structures to avoid potential serious bleeding complications. For percutaneous closure of large arteriotomies several techniques could be used. Arteriotomy closure with the Prostar™ (Abbott Vascular Devices, Redwood City, CA, USA) system was deemed possible; in case of closure device failure additional sutures could be installed with a Proglide™ (Abbott Vascular) system as long as the wire access is secured. Surgical back up was guaranteed by a cardiothoracic surgeon scrubbed in on the case.
The procedure was performed under general anaesthesia. The head was hyperextended in a left lateral position with optimal exposure of the right supra- and infraclavicular triangles. With ultrasound assessment (high resolution Siemens X 300 Echo machine and a Siemens 7-10 Mhz linear probe; Siemens, Erlangen, Germany) the axillary artery was located precluding any anterior venous interference and preserving an adequate interspace to the acromioclavicular joint to guarantee enough manoeuvrability of the device systems during the procedure (Figure 2, Moving image 1). Under real-time ultrasonic guidance an 18 G needle was introduced into the axillary artery, and with a standard Seldinger technique a 6 Fr sheath was inserted. A 190 cm J-tipped hydrophilic Terumo wire was then advanced into the descending aorta. A 1 cm incision was made with a scalpel, followed by blunt dissection of the subcutaneous tissue up to the axillary artery. A Prostar ™ (Abbott Vascular Devices) preclosure was obtained and a 35 cm 18 Fr Solopath™ arterial sheath (Onset Medical Corporation, Irvine, CA, USA) was introduced (Moving image 2). A Solopath sheath was selected for its superior kink resistance and low profile during its introduction into the vessel. The implantation of a 31 mm Medtronic CoreValve™ (Medtronic, Minneapolis, MN, USA) was then executed according to standard TAVI techniques. After confirmation of proper Medtronic CoreValve System position and function with no residual transvalvular gradient and only mild paravalvular leakage, percutaneous access site closure was performed (Figure 3, Moving image 3). A 260 cm Amplatz Super Stiff™ guidewire (Boston Scientific Corp., Natick, MA, USA), was re-advanced into the descending aorta to preserve access while retracting the 18 Fr sheath and allow for smooth reinsertion of an 18 Fr sheath for haemostasis and optimal surgical exploration in case of closure device failure, bleeding or major vascular complication (Moving image 4). After confirmation of adequate haemostasis, the safety wire was retracted and the knots were closed on the arteriotomy (Figure 4 and Figure 5).
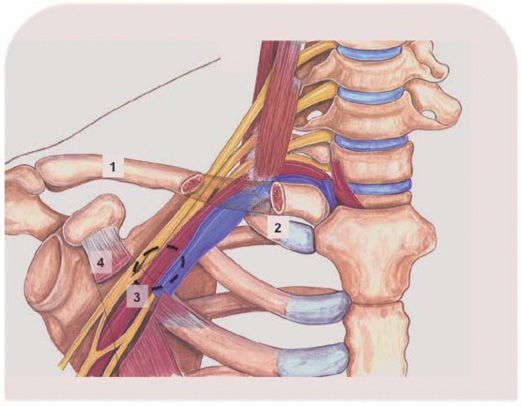
Figure 2. Schematic representation of the anatomical interrelationship between the vascular bundle and the clavicle of the right shoulder. The clavicle has been made transparent where it overlies the vascular bundle. The vein is blue, the artery is red. Dotted area marks optimal axillary artery puncture site without overlying venous interference. 1: clavicle; 2: first rib; 3: axillary artery; 4: minor pectoral muscle
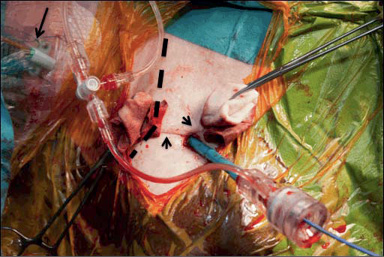
Figure 3. Skin incision with the 18 Fr Solopath™ sheath in situ. Note the Prostar™ suture wires surrounding the sheath (arrowheads). A venous sheath (arrow) is introduced into the right internal jugular vein. Dotted shaded area marks the right clavicle.
Figure 4. The 31 mm Medtronic CoreValve in situ with only trivial residual paravalvular aortic regurgitation.
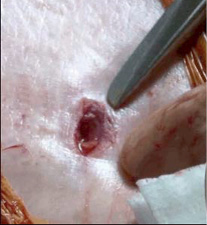
Figure 5. Skin incision at the end of the procedure after arteriotomy closure. The index finger and scissors mark as reference.
We describe for the first time a completely percutaneous transaxillary TAVI in human. Real-time multimodality imaging allowed for a well-controlled and safe arterial access with minimal tissue damage. Multidisciplinary planning and execution with active participation of interventional cardiologists, cardiothoracic surgeons, anaesthesiologists and imaging specialist is key. Until now the transfemoral arterial approach was the only truly percutaneous strategy for TAVI. In case of an unfavourable femoral-Iliac anatomy, alternative access strategies required surgical techniques. Our case underscores the evolving concept to explore minimally invasive treatment options for patients with AS at high operative risk and offers a full-fledged percutaneous alternative to transfemoral TAVI.
Conflict of interest statement
The authors have no conflict of interest to declare.
Online data supplement
Moving image 1. Echo guided puncture into the right axillary artery.
Moving image 2. Deployment of the Solopath™ to its maximal diameter.
Moving image 3. Final angiogram after Medtronic CoreValve implantation.
Moving image 4 A and B. Solopath™ sheath retraction and arteriotomy closure by pulling on the Prostar™ sutures.

