Abstract
Over the last decade, transcatheter aortic valve implantation (TAVI) has gained widespread acceptance for the treatment of high surgical risk or inoperable patients with severe aortic stenosis. Spurred on by initial success and an ever-growing body of supporting data, TAVI has undergone rapid technological advancements in recent years with a focus on procedural simplification and limiting complications associated with the early devices. In this article, we provide a brief overview of the past, current and newer devices for transfemoral (TF) TAVI and their post-procedural outcomes.
Introduction
Transcatheter aortic valve implantation (TAVI) has become the new standard of care for high surgical risk or inoperable patients with severe aortic stenosis, affording excellent periprocedural, early and midterm outcomes. The majority of TAVI procedures performed in the initial decade after its introduction in 20021 used one of the first-generation devices: the balloon-expandable Edwards SAPIEN and SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA) and the self-expanding CoreValve® (Medtronic, Minneapolis, MN, USA). These devices demonstrated outcomes which were superior to medical therapy and comparable to surgery in randomised trials. However, concerns including procedural complexity, paravalvular regurgitation, valve malpositioning, lack of repositionability and retrievability, neurological complications and conduction disturbances limited the expansion of TAVI to intermediate surgical risk and other population subsets. The favourable outcomes and accompanying limitations observed with the first-generation devices fuelled subsequent rapid innovations and refinements in device designs and delivery system technologies that have resulted in several second-generation devices receiving a CE mark in recent years (Table 1). Several other devices which have been specifically designed to overcome the limitations of first-generation devices are in early clinical evaluation but have not yet received their CE mark. All currently available and new-generation devices can be broadly grouped based upon their mode of deployment: balloon-expandable, self-expanding or differential device deployment (the latter two groups being partially or fully repositionable) (Figure 1). In this focused article, we provide an overview of these valve technologies and their recent clinical evidence base when used for TAVI via the transfemoral (TF) route – the most frequently used access site for valve implantation.
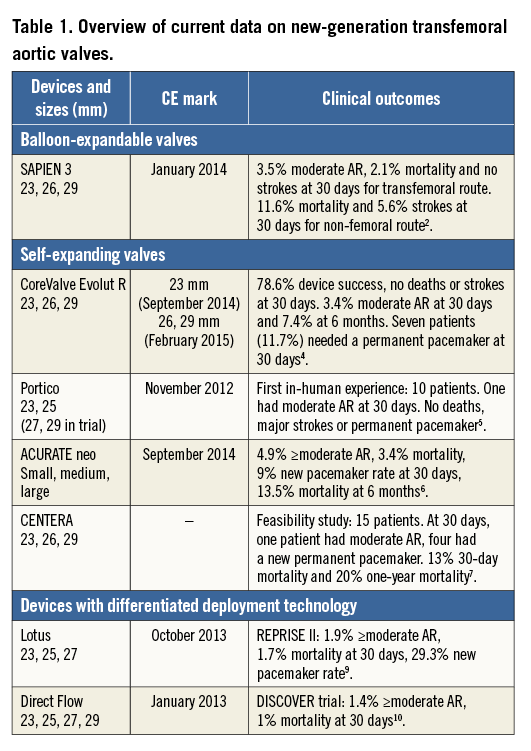
Figure 1. Devices for transfemoral transcatheter aortic valve implantation (TAVI).
Balloon-expandable valves
Conceptually, all balloon-expandable valves consist of biological cardiac valve tissue sewn inside an expandable stent frame, which is subsequently crimped onto a balloon whose expansion results in valve deployment. Deployment requires rapid ventricular pacing.
THE EDWARDS SAPIEN EVOLUTION: CRIBIER-EDWARDS, EDWARDS SAPIEN, SAPIEN XT AND SAPIEN 3
The TAVI clinical experience began with the Cribier-Edwards balloon-expandable valve (Edwards Lifesciences), which consisted of equine pericardium and a stainless steel frame1. Modification of this valve resulted in the Edwards SAPIEN valve, which also consisted of a stainless steel frame, but with bovine pericardial valve tissue and a fabric skirt made of polyethylene terephthalate (PET) in order to improve sealing. The RetroFlex delivery system (Edwards Lifesciences) accompanying this device was of large diameter (22-24 Fr) and associated with higher rates of vascular complications after TF implantation. The Edwards SAPIEN XT was then introduced with a cobalt-chromium stent frame, fewer and thinner struts and a low-profile NovaFlex delivery system (Edwards Lifesciences) to reduce vascular complications (Figure 2).
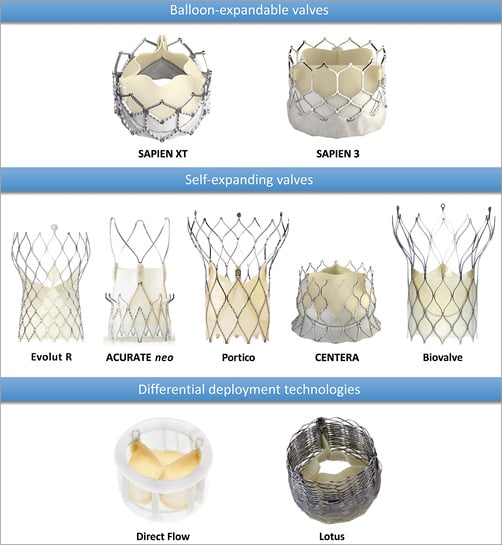
Figure 2. Current transfemoral TAVI devices. Pictures provided courtesy of Edwards Lifesciences, Irvine, CA, USA; Medtronic, Minneapolis, MN, USA; Symetis SA, Ecublens, Switzerland; St. Jude Medical, St. Paul, MN, USA; Biotronik AG, Bülach, Switzerland; Direct Flow Medical Inc., Santa Rosa, CA, USA and Boston Scientific, Marlborough, MA, USA.
The SAPIEN 3 valve (Edwards Lifesciences) is the latest generation of the Edwards SAPIEN family and consists of a trileaflet pericardial bovine valve mounted in a cobalt-chromium stent with an additional outer PET cuff to further enhance paravalvular sealing (Figure 2). The highly flexible, low-profile TF Commander delivery system (Edwards Lifesciences) has a distal short tapered tip to facilitate crossing of the native valve as well as additional features to facilitate valve alignment and positioning. The device is introduced through a 14/16 Fr expandable sheath (eSheath; Edwards Lifesciences), which transiently expands to accommodate the device before returning to its lower profile.
In a multicentre study of SAPIEN 3 valve implantations, rates of moderate paravalvular regurgitation at 30 days were as low as 3.5%. TF implantation was associated with lower mortality and stroke rates as compared to alternative access (Table 1)2. Studies comparing the outcomes of different generations of SAPIEN valves have been few and several analyses are ongoing (e.g., CHOICE Extend). In a propensity score-matched, single-centre analysis, SAPIEN 3 was associated with significantly lower rates of ≥mild paravalvular regurgitation (15.9% versus 46.2%, p=0.003) before hospital discharge compared to SAPIEN XT. No differences in pacemaker rates (9.8% versus 8.8%, p=0.94) and 30-day mortality (both 5%) were observed3.
Self-expanding valves
These valves consist of biological cardiac valve tissue sewn inside a self-expanding nitinol-based stent frame, which is subsequently crimped inside a delivery capsule whose withdrawal results in valve deployment.
THE COREVALVE EVOLUTION: COREVALVE AND EVOLUT R
The CoreValve device (Medtronic) is the prototype of self-expanding devices and consists of bovine (first-generation device, 25 Fr delivery sheath) or porcine (second- and third-generation, 21 Fr and 18 Fr delivery sheaths, respectively) pericardial tissue mounted on a nitinol frame. The lower profiles of the newer-generation devices were achieved by switching to porcine pericardium and using a more flared outflow design. The stent frame consists of an inflow segment, a narrower supra-annular middle segment containing the leaflets, and an outflow segment with a cell design that allows blood flow and catheter access to the coronary ostia. A delivery catheter modification employed the low-profile AccuTrak™ delivery system (Medtronic) with features to reduce frictional forces during valve deployment.
The CoreValve Evolut R™ (Medtronic) is the latest in the family of CoreValve devices and has several modifications that make it repositionable, resheathable and recapturable. It is shorter in height than its predecessor but retains the 12 mm height of the pericardial skirt (Figure 2). Additionally, leaflets are treated with alpha-amino oleic acid, which binds to aldehyde groups within the pericardial tissue to inhibit calcification. The valve comes with its own EnVeo™ R delivery system (Medtronic), a very low-profile sheath (14 Fr equivalent) and the promise of first-time deployment accuracy4.
THE PORTICO VALVE
The Portico™ device (St. Jude Medical, St. Paul, MN, USA) is a resheathable, repositionable and retrievable prosthesis consisting of bovine pericardial tissue and a nitinol frame (Figure 2). The inflow portion is made of porcine pericardium and functions as a sealing cuff while the leaflets are placed at the annular position close to the ventricular end. A lower leaflet profile and a more vertical ventricular stent end minimise left ventricular outflow tract protrusion and reduce the risk of conduction disturbances. The large open cell design of the stent frame preserves coronary flow and access. A feasibility study in 10 patients showed no incidence of stroke, new pacemaker implantation or death at 30 days after implantation and only one patient with moderate aortic regurgitation5.
THE ACURATE NEO VALVE
The ACURATE neo™ (Symetis, Ecublens, Switzerland) device consists of a porcine aortic root valve and a nitinol frame with an hourglass shape containing three stabilisation arches which self-position the device during a “top-down” 3-step deployment (Figure 2). The valve leaflets are placed in a supra-annular position in the upper crown segment of the stent and the annular portion of the valve has a PET sealing skirt to reduce paravalvular regurgitation (refer to Table 1 for clinical results)6.
THE CENTERA VALVE
The CENTERA valve (Edwards Lifesciences) is a self-expanding, ultra low-profile valve composed of a nitinol frame and bovine pericardium, which can be delivered by a single operator using a 14 Fr motorised delivery system. The stent frame is shorter than other self-expanding valves, and the ventricular end is flared to reduce left ventricular protrusion (Figure 2). The flaring of the ventricular portion was reduced in subsequent models because of increased conduction disturbances observed in early studies7. Unlike other self-expanding valves, the CENTERA valve is not functional until fully deployed and therefore requires rapid ventricular pacing at the time of implantation. The CE-mark approval study is ongoing.
THE BIOVALVE
The resheathable and repositionable Biovalve (Biotronik AG, Bülach, Switzerland) features a porcine pericardial valve mounted on a nitinol stent and is delivered through an 18 Fr delivery system with a single user-friendly component for positioning, resheathing and valve release (Figure 2). The valve has larger stent cell size in its outflow portion to facilitate coronary access. Implantation feasibility and short-term safety were demonstrated in the recently published BIOVALVE-1 study8.
Differential deployment technologies
THE LOTUS VALVE
The Lotus™ valve (Boston Scientific, Marlborough, MA, USA) consists of bovine pericardial valve tissue mounted on a braided nitinol stent with an outer adaptive seal (Figure 2). The valve is pre-attached to the delivery system, which has two controls (a control knob and a release collar). The control knob facilitates complete repositioning and redeployment prior to final release, which is achieved by activation of the release collar. Valve deployment is achieved by mechanical expansion utilising the interaction of posts and buckles that are connected to the inner catheter of the delivery system. In the REPRISE II trial, device success was nearly 100% with only one patient having moderate aortic regurgitation at 30 days9. High rates of new pacemaker implantation were attributed to device oversizing as a consequence of availability in only two sizes (23 and 27 mm).
THE DIRECT FLOW MEDICAL VALVE
The Direct Flow Medical® valve (Direct Flow Medical, Santa Rosa, CA, USA) is a non-metallic valve consisting of bovine pericardial tissue mounted over an inflatable two-ring structure covered with polyester fabric (Figure 2). These rings are able to adapt their shape to the native aortic annulus in order to prevent paravalvular regurgitation and can also be deflated to reposition the prosthesis if required. Once optimal positioning is achieved, an epoxy-based curing polymer is used to fill the structure, which subsequently solidifies to provide permanent support and position. The device may be difficult to position in patients with extensive outflow tract calcification. Safety and performance were evaluated in the DISCOVER trial where freedom from mortality and adverse events at 30 days were 99% and 91%, respectively10.
Comparison studies of devices with different designs
A plethora of TAVI devices is now available as a result of rapid technological evolution, each with its own advantages and clinical outcomes. As a consequence, it is now essential to obtain data comparing the available devices in order to guide appropriate device selection.
To date, the CHOICE trial is the only randomised head-to-head study comparing balloon- and self-expandable TAVI11. In this investigator-initiated trial, the use of a balloon-expandable device resulted in a significantly higher rate of device success and a lower rate of moderate or severe paravalvular regurgitation. The need for a new pacemaker was also lower in the balloon-expandable group at 30 days. At one-year follow-up, there were no significant differences between the two groups with regard to mortality (cardiovascular or all-cause), stroke and repeat hospitalisation for heart failure. More than mild paravalvular regurgitation was more frequent in the self-expanding group, as observed at 30 days. Of concern, four patients in the balloon-expandable group had probable valve thrombosis as opposed to none in the self-expanding group12.
These key findings have been replicated in several recent publications from multicentre registries. Six-year follow-up data on 3,980 TAVI procedures performed in the United Kingdom from 2007-2012 showed no difference in survival at any time point between SAPIEN and CoreValve devices13. Use of the self-expanding CoreValve device was associated with a significantly higher incidence of aortic regurgitation and need for pacemaker implantation. Similarly, in a recent meta-analysis comparing the valve types, self-expanding valve implantation was associated with a significantly higher incidence of new pacemaker implantation, ≥2+ aortic regurgitation at 30 days, valve embolisation, and the need for >1 valve, although one-year mortality was similar with both self-expanding and balloon-expandable valves following TAVI via the TF approach14.
There are currently no published randomised trials comparing newer-generation devices. In a matched comparison of high surgical risk patients undergoing TAVI, use of the mechanically expanded Lotus valve was associated with higher rates of device success compared with the self-expanding CoreValve, a finding driven principally by higher rates of correct anatomic positioning and lower rates of moderate paravalvular regurgitation15. Cardiovascular mortality (0% for Lotus versus 4%), and rates of major stroke (4% for Lotus versus 2%) and permanent pacemaker insertion (28% for Lotus versus 18%) did not differ at 30 days (all p=NS). In a non-randomised trial comparing Lotus and SAPIEN 3 valves, post-procedural and other 30-day outcomes were similar with the two devices. There were no cases of more than mild aortic regurgitation with both devices. However, the Lotus valve was associated with a significantly higher need for permanent pacemaker implantation (26.9% versus 3.8% for SAPIEN 3, p<0.003)16. Several randomised head-to-head approval trials are currently underway in the USA.
Conclusion
TAVI is an established alternative to surgery in inoperable and high-risk patients with severe aortic stenosis and a wide range of devices is now available. In recent years, TAVI technology has undergone rapid innovation, with development of devices specifically designed to address previous concerns regarding paravalvular regurgitation, conduction disturbances and vascular complications. Refinements of the design and features of the new prostheses include improved annular conformability, better valve sealing, and repositionability, which have virtually eliminated many of the limitations of first-generation devices. Preliminary data with these new devices are encouraging, although clinical experience is limited and long-term data are required before TAVI can be routinely offered to low- and intermediate-risk subjects.
Conflict of interest statement
M. Abdel-Wahab and G. Richardt have received institutional research grants from St. Jude Medical and Biotronik. M. Abdel-Wahab is a proctor for Boston Scientifc. G. Richardt receives lecture fees from Edwards Lifesciences and Boston Scientific. J. Jose is currently supported by an EAPCI grant in Interventional Cardiology which is partially sponsored by Medtronic.

