
Abstract
The presence of a patent foramen ovale (PFO) is implicated in the pathogenesis of a number of medical conditions; however, the subject remains controversial and no official statements have been published. This interdisciplinary paper, prepared with involvement of eight European scientific societies, aims to review the available trial evidence and to define the principles needed to guide decision making in patients with PFO. In order to guarantee a strict process, position statements were developed with the use of a modified grading of recommendations assessment, development, and evaluation (GRADE) methodology. A critical qualitative and quantitative evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk/benefit ratio. The level of evidence and the strength of the position statements of particular management options were weighed and graded according to predefined scales. Despite being based often on limited and non-randomised data, while waiting for more conclusive evidence, it was possible to conclude on a number of position statements regarding a rational general approach to PFO management and to specific considerations regarding left circulation thromboembolism. For some therapeutic aspects, it was possible to express stricter position statements based on randomised trials. This position paper provides the first largely shared, interdisciplinary approach for a rational PFO management based on the best available evidence.
Abbreviations
AF: atrial fibrillation
AUC: area under the receiver operating curve
c-TCD: contrast-enhanced transcranial Doppler
c-TOE: contrast transoesophageal echocardiography
c-TTE: contrast-enhanced transthoracic echocardiography
DOAC: direct oral anticoagulants
DVT: deep vein thrombosis
ECG: electrocardiogram
GRADE: Grading of recommendations assessment, development, and evaluation
ICM: insertable cardiac monitors
LAE: left atrium enlargement
LVH: left ventricle hypertrophy
NNH: number needed to harm
NNT: number needed to treat
OAC: oral anticoagulants
OR: odds ratio
OSAS: obstructive sleep apnoea syndrome
PE: pulmonary embolism
PICO: population-intervention-comparator-outcome
PFO: patent foramen ovale
RCT(s): randomised clinical trial(s)
RoPE: risk of paradoxical embolism
R-T-L: right-to-left
Rx: therapy
TIA: transient ischaemic attack
Introduction
The presence of a patent foramen ovale (PFO) is implicated in the pathogenesis of a number of medical conditions. Recent randomised clinical trials (RCTs) have shown evidence of benefit for device closure as compared with medical therapy in patients with cryptogenic stroke. However, we are rarely able to be categoric about the role of PFO in any given clinical setting, stressing the need for specific clinical and research approaches for complex scenarios1-5. Moreover, most studies on the subject are observational, with an ensuing low certitude of effects and very disparate, often contradictory, clinical choices in different local realms in the absence of official positions. To address these concerns, the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Scientific Documents and Initiatives Committee invited eight European scientific societies and international experts to develop shared and rational position statements on the management of PFO to help clinicians in decision making. To address that request, this paper aims to define interdisciplinary rational principles needed to guide management of patients with PFO by using a strict methodology to prepare position statements with different underlying quality of evidence, based on systematic literature reviews for each of the considered issues and performing quantitative assessments whenever possible.
The present paper reports the approach to patients with PFO and left circulation thromboembolisms, that affect large numbers of patients6-8. A subsequent paper will report on decompression sickness, desaturation syndromes, migraine, and other clinical settings.
Methods
In order to guarantee a strict evidence-based process, position statements were developed with the use of a modified grading of recommendations assessment, development, and evaluation (GRADE) methodology (http://gdt.guidelinedevelopment.org/app/handbook/handbook.html), by answering population-intervention-comparator-outcome (PICO) questions and non-PICO questions.
A detailed review of the methodology used can be found in Supplementary Appendix 1, Supplementary Appendix 2, Supplementary Appendix 3 and Supplementary Table 12. Systematic reviews and statistical analysis were performed by a dedicated evidence synthesis team.
IS PFO ASSOCIATED WITH CRYPTOGENIC LEFT CIRCULATION THROMBOEMBOLISM?
The association between PFO and cryptogenic left circulation thromboembolism has mainly been addressed in studies including cryptogenic stroke and is strongly supported by epidemiological data9-13, clinical observational studies14-25 (Supplementary Appendix 4) and by RCTs showing that PFO closure reduces stroke recurrence in comparison with medical therapy26-29.
However, the evidence has been controversial due to the different role that a PFO can play in different clinical scenarios and to the lack of adequately dimensioned prospective studies. Pathophysiological processes include paradoxical embolism, thrombus forming within the PFO, left atrial dysfunction, and atrial arrhythmias (Supplementary Appendix 4). Research aimed at identifying individual patients’ phenotypes is needed to improve clinical management.
DEFINITIONS OF PFO-RELATED LEFT CIRCULATION THROMBOEMBOLISM
PFO has been associated with left circulation thromboembolism to several organs30; therefore we promote the use of standardised definitions.
Cryptogenic ischaemic left circulation embolisms are defined as any definite ischaemia (symptomatic or asymptomatic) occurring in an arterial bed which lacks a known cause despite investigation. Patients presenting with this clinical picture should be screened for the presence or absence of a PFO. However, when a PFO is thought likely to be implicated in a cryptogenic embolism, the event should be classified as PFO-related instead of cryptogenic31. Current classifications do not yet generally include this aspect32-35.
GENERAL APPROACH TO PFO MANAGEMENT
The management we propose in this paragraph applies to systemic thromboembolism as well as to all PFO-associated syndromes. An overview of the general approach to PFO management is summarised in Table 1.
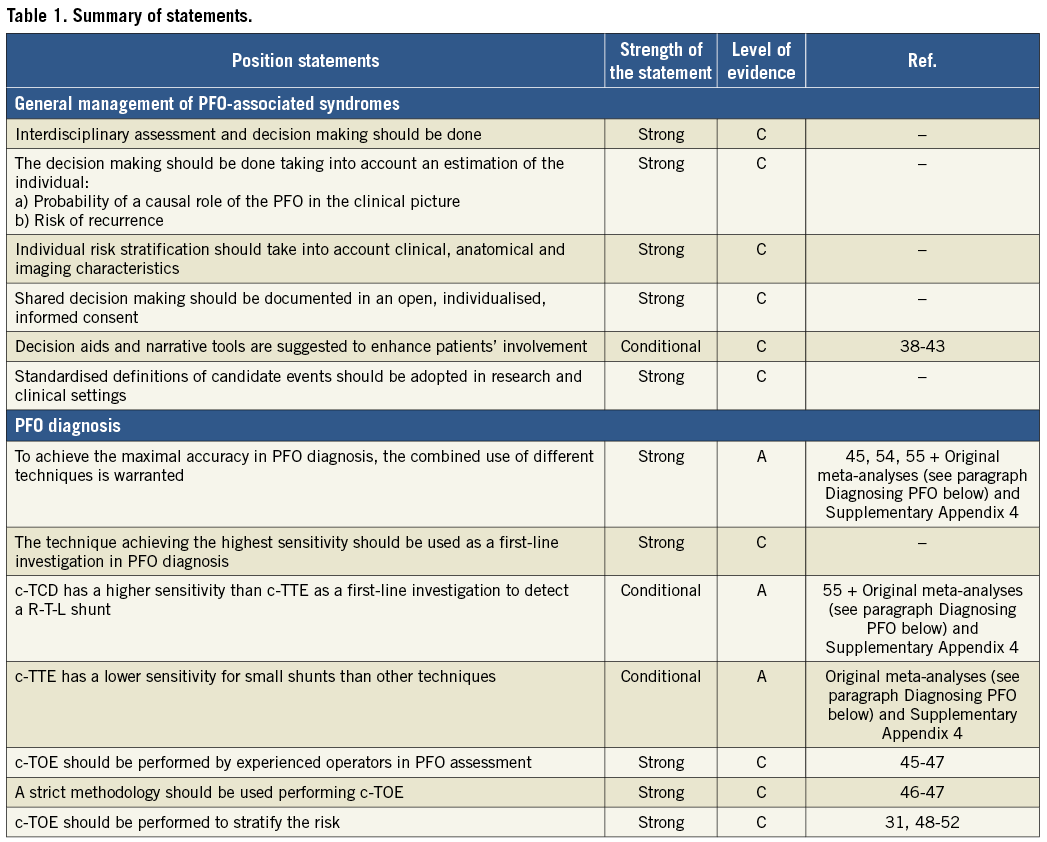
THE MAIN AXES OF EVALUATION
In all clinical scenarios, the two main axes guiding assessment and treatment of PFO should be: 1) the probability that any PFO has a relevant role in the observed clinical picture; 2) the likelihood that the observed clinical event will recur. For patients with the highest probability of both, closure of the PFO should be advised. For patients with the lowest probability, medical therapy should be considered. For patients with intermediate probabilities, clinical judgement is required to allow good decision making in liaison with the patient.
PROACTIVE APPROACH: AN INTERDISCIPLINARY COLLABORATION, SHARED DECISION MAKING, AND OPEN INFORMED CONSENT
Interdisciplinary involvement in decision making regarding PFO management is axiomatic and should include an interventional cardiologist and other specialists dictated by the patient’s clinical manifestations. Active involvement of the patient in the decision-making process is mandatory36,37 and should be documented in an individualised, open, informed consent. The development of specific decision aids and the use of narrative tools are encouraged38-43.
DIAGNOSING PFO
The diagnosis of PFO is required only for deciding on a treatment. Several techniques can be used to diagnose PFO44. Their characteristics are summarised in Supplementary Table 1. High-quality comparative studies are still needed to express a conclusive position on the best diagnostic strategies.
Contrast transoesophageal echocardiography (c-TOE) provides unparalleled visualisation of the interatrial septum and other relevant structures and can show the shunt itself. A meta-analysis of the accuracy of c-TOE in the diagnosis of PFO compared to autopsy, cardiac surgery, and/or catheterisation yielded a weighted sensitivity of only 89%45. Inability to perform an adequate Valsalva manoeuvre during transoesophageal echocardiography is probably responsible46,47 (Supplementary Figure 1). Nonetheless, c-TOE is necessary to characterise the PFO and stratify the risk in the diagnostic phase31,48-52, and systematic reporting of a set of parameters could help in guiding assessment (Table 2). Bleeding, aspiration, or oesophageal perforation are rare TOE complications53.
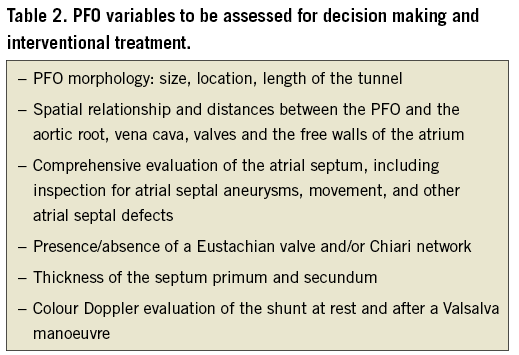
In our updated meta-analysis of 29 studies comparing contrast-enhanced transcranial Doppler (c-TCD) with c-TOE across 2,751 patients (Supplementary Appendix 3, Supplementary Appendix 4, Supplementary Figure 19), c-TCD had a sensitivity of 94% and a specificity of 92% (Supplementary Figure 2A) with an area under the receiver operating curve (AUC) of 0.97 (Supplementary Figure 2B). This meta-analysis was limited by the low quality of evidence (Supplementary Table 2) and by the inconsistency across studies, being 67% for sensitivity and 73% for specificity. In a previous meta-analysis, the specificity of c-TCD was increased to 100% when the threshold for a positive shunt was increased to 10 high-intensity transient signals54.
We also performed an original meta-analysis of 13 studies across 1,360 patients comparing contrast-enhanced transthoracic echocardiography (c-TTE) against c-TOE (Supplementary Appendix 3, Supplementary Appendix 4, Supplementary Figure 20). c-TTE was only 88% sensitive and 82% specific with an AUC of 0.91 (Supplementary Figure 3A), a severe inconsistency among studies (Supplementary Figure 3B) and a low quality of evidence (Supplementary Table 2). A recent meta-analysis also showed superior overall diagnostic yield of c-TCD compared to c-TTE55.
At present, grounded on the accrued low-quality evidence, no technique can be considered a gold standard and, in most cases, a precise diagnosis of PFO needs the combined use of different techniques, prescribed according to their different characteristics. As first-line investigations must warrant accuracy by minimising false negative screenings, we propose a diagnostic algorithm in Figure 1 that can be adapted to satisfy disparate clinical and logistic needs.
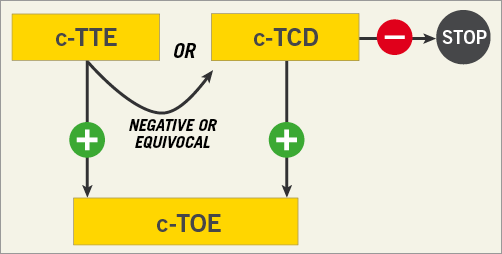
Figure 1. Algorithm for the diagnosis of PFO. c-TCD: contrast-enhanced transcranial Doppler; c-TOE: contrast-enhanced transoesophageal echocardiography; c-TTE: contrast-enhanced transthoracic echocardiography; –negative test for the presence of right-to-left shunt; +positive test for the presence of right-to-left shunt
ASSESSMENT OF THE ROLE OF A PFO IN LEFT CIRCULATION EMBOLISM
A PFO is seen in ~25% of the general population and may therefore coexist by chance in a patient with an unexplained left circulation embolism. Due to the complexity and number of the variables influencing the process, and the low scientific quality of the related literature, no position can be expressed regarding the assessment of the role of a PFO in a quantitative way; therefore, this role should be evaluated with critical clinical judgement in an interdisciplinary collaboration between physicians, weighting the following different features on an individual basis. For a more detailed discussion of each of the following paragraphs please refer to Supplementary Appendix 4. Position statements are summarised in Table 3.
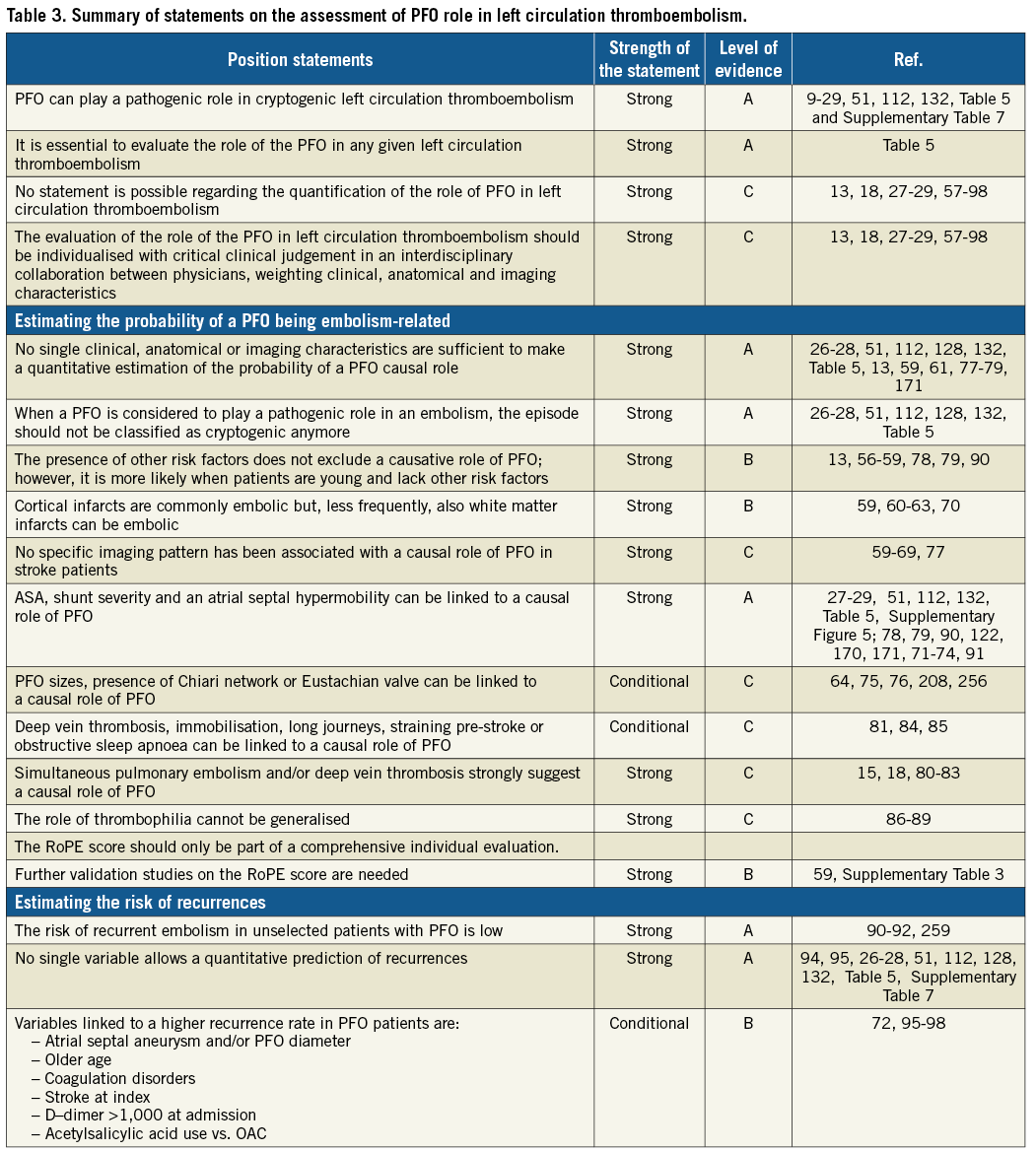
IS IT POSSIBLE TO ESTIMATE THE LIKELIHOOD OF A PFO-MEDIATED STROKE?
Patient characteristics
A meta-analysis of observational studies showed a stronger relative association of PFO with cryptogenic stroke in patients <55 years as compared to older patients56. However, the association was also observed in older patients13,57,58. The presence of other comorbidities or clinical risk factors for stroke does not, per se, exclude a pathophysiological role of PFO in cryptogenic embolism, though their absence increases the likelihood of its pathogenic role59.
Imaging stroke pattern
Neither the localisation nor type of infarct pattern in grey or white matter was specific for PFO embolism in observational studies59-69. Cortical infarcts are usually considered embolic but a recent patient-level meta-analysis of RCTs plausibly suggests that non-cortical infarcts can also have an embolic origin70.
Characteristics of the PFO
An atrial septal aneurysm (ASA) and/or a moderate-to-severe shunt were strongly associated with a causal role of PFO in patients with cryptogenic stroke in observational and randomised studies27-29,71-74. Other characteristics associated in randomised studies with a causal PFO are large PFO size and atrial septal hypermobility29. A Eustachian valve, Chiari network or a long PFO tunnel was suggested to be linked to PFO-associated strokes but only in retrospective studies75,76. Other studies have failed to detect one or more of these associations, however59,77-79, underlining the heterogeneity of phenotypes and the need to identify them.
Clinical clues
Candidate clinical clues have been addressed in retrospective studies and infrequently in prospective observational studies. Logically, conditions that strongly suggest paradoxical embolism in the presence of a PFO include the simultaneous or previous occurrence of pulmonary emboli18,80,81 or the documentation of a venous source of embolism around the time of stroke. Absence of evidence of venous thrombus is unhelpful because of frequent false negatives15,80,82,83 but immobilisation, recent major surgery, or an extended car or airplane journey implies possible venous clot development. Activity at the time of the stroke is also relevant – straining manoeuvres, obstructive sleep apnoea with stroke-on-waking should be enquired for81,84,85. Retrospective studies that have attempted to identify an association between inherited thrombophilia and PFO-related stroke have yielded conflicting results86-89.
The risk of paradoxical embolism (RoPE) score represents an attempt to assign a causal relationship probability to individual PFOs in the setting of stroke of unknown cause59 and may be useful in helping to guide management decisions. However, it should always be used in conjunction with other parameters because the quality of evidence of internal validation studies has been rated moderate at best (Supplementary Figure 15, Supplementary Table 3), and no large external validation studies have been published.
In addition, the RoPE score does not account for high-risk PFO features (e.g., septal aneurysm) that have been shown to correlate with higher risk of paradoxical embolisation.
WHAT IS THE RISK OF RECURRENCE IN A PFO-ASSOCIATED STROKE?
Meta-analyses of observational and/or randomised studies suggest that the annual recurrence rate on medical therapy ranges from 0% to 5.8% for stroke and from 0% to 14% for either stroke or transient ischaemic attack (TIA)90-92. This wide variability stresses the heterogeneity of phenotypes in these syndromes. Causes of recurrence can of course include non-PFO mediated mechanisms93,94.
Some predictors of stroke recurrence have been identified prospectively and retrospectively72,95-97 (Supplementary Figure 6). Supplementary Table 4 lists features that were statistically significant predictors in at least two studies. Atrial septal aneurysm anatomy is particularly predictive (Supplementary Appendix 4). In one study98, a high D-dimer level on admission was an independent predictor of recurrent ischaemic stroke in patients with PFO. Therefore, at present, the individual evaluation of the risk of recurrence also cannot be quantitatively scored and should be based on interdisciplinary qualitative clinical evaluation.
UNIFIED DIAGNOSTIC WORKUP IN LEFT CIRCULATION THROMBOEMBOLISM
A diagnostic workup should follow logical steps (Figure 2). Table 4 summarises position statements. Further details are provided in Supplementary Appendix 4.
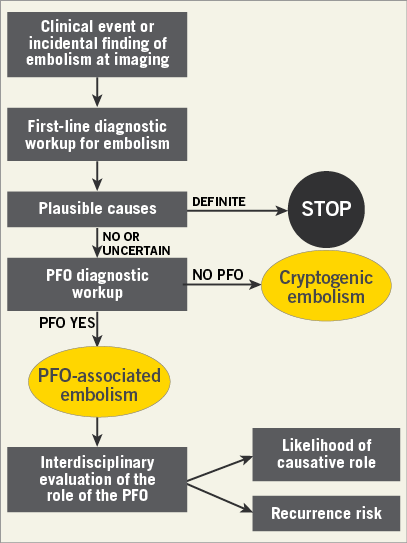
Figure 2. Algorithm for the diagnostic workup of cryptogenic left circulation thromboembolism.
The diagnostic process should always include interdisciplinary clinical assessments and appropriate imaging.
Identifying atrial fibrillation (AF) is important because recurrences of left circulation embolism are, in the majority of cases, due to left atrial appendage thrombosis instead of paradoxical embolism. However, AF can be difficult to detect. A routine 12-lead electrocardiogram (ECG) and either in-patient cardiac telemetry or 24-hour Holter monitoring are sufficient to diagnose permanent AF and sufficiently long transient AF episodes. However, randomised and observational studies showed that insertable cardiac monitors (ICM) are associated with an increased yield of paroxysmal AF diagnoses relative to standard monitoring also in cryptogenic stroke99-104 (Supplementary Appendix 4, Supplementary Figure 16). Therefore, in high-risk patients for AF, an ICM period of six months can be reasonably considered to rule out AF before deciding on PFO closure105. In Figure 3 we propose a strategy based on risk stratification of patients to be applied with a critical clinical judgement (Supplementary Appendix 4). During ICM monitoring, patients should be maintained on medical therapy (see below). After six months, whatever the chosen treatment, the monitoring can be extended to the full duration of the ICM life to identify episodes of paroxysmal AF106-112, to monitor the atrial thrombosis burden in arrhythmic patients, and to aid diagnosis in case recurrent ischaemia occurs.
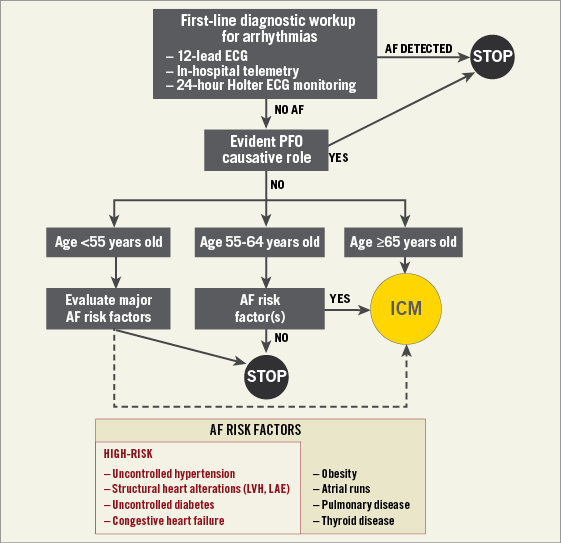
Figure 3. Flow chart for the screening of overt atrial fibrillation in cryptogenic left circulation thromboembolism. The cut-off ages of 55 and 65 years old have been chosen according to data from large epidemiological studies166,173. Patients <55 years may be considered for ICM when they have high clinical suspicion of AF (i.e., ≥2 high-risk factors for AF). ECG: electrocardiography; LAE: left atrium enlargement; LVH: left ventricle hypertrophy
MEDICAL AND INTERVENTIONAL MANAGEMENT
Further insights on each of the following paragraphs can be found in Supplementary Appendix 4.
PICO questions for the choice of therapy are summarised in Table 5 and Supplementary Appendix 5. Figure 4 summarises the flow of the choice of the therapy.
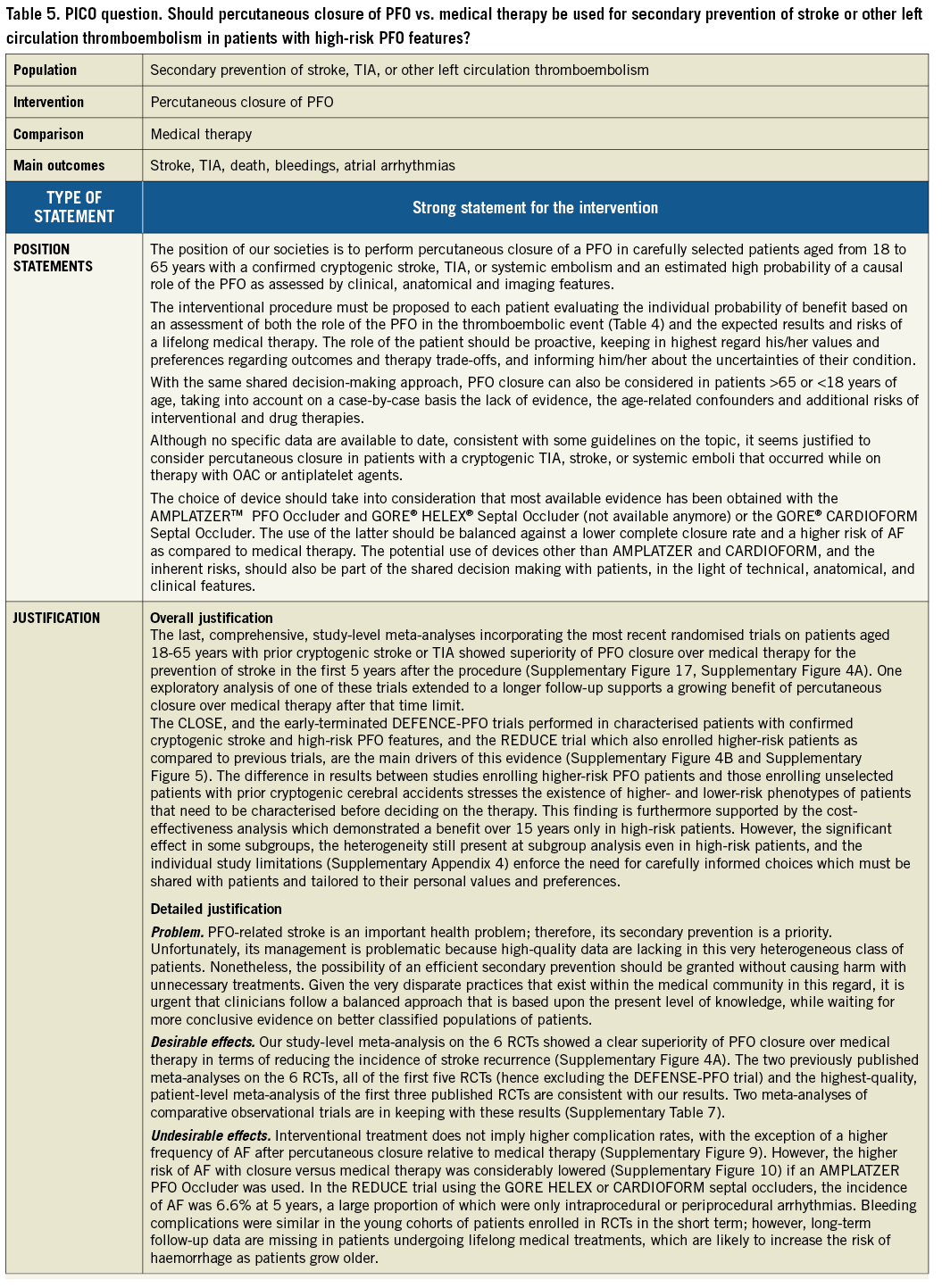
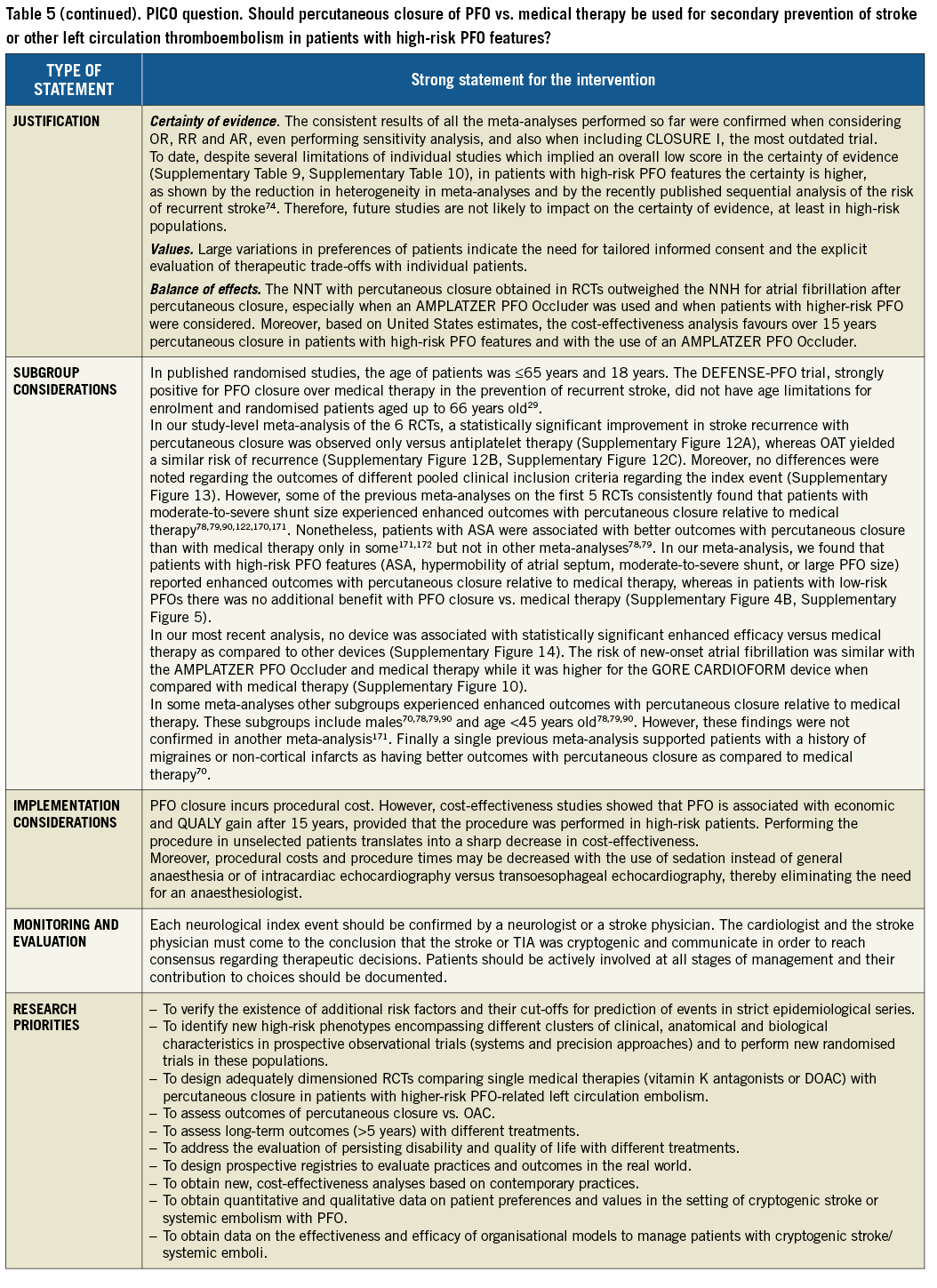
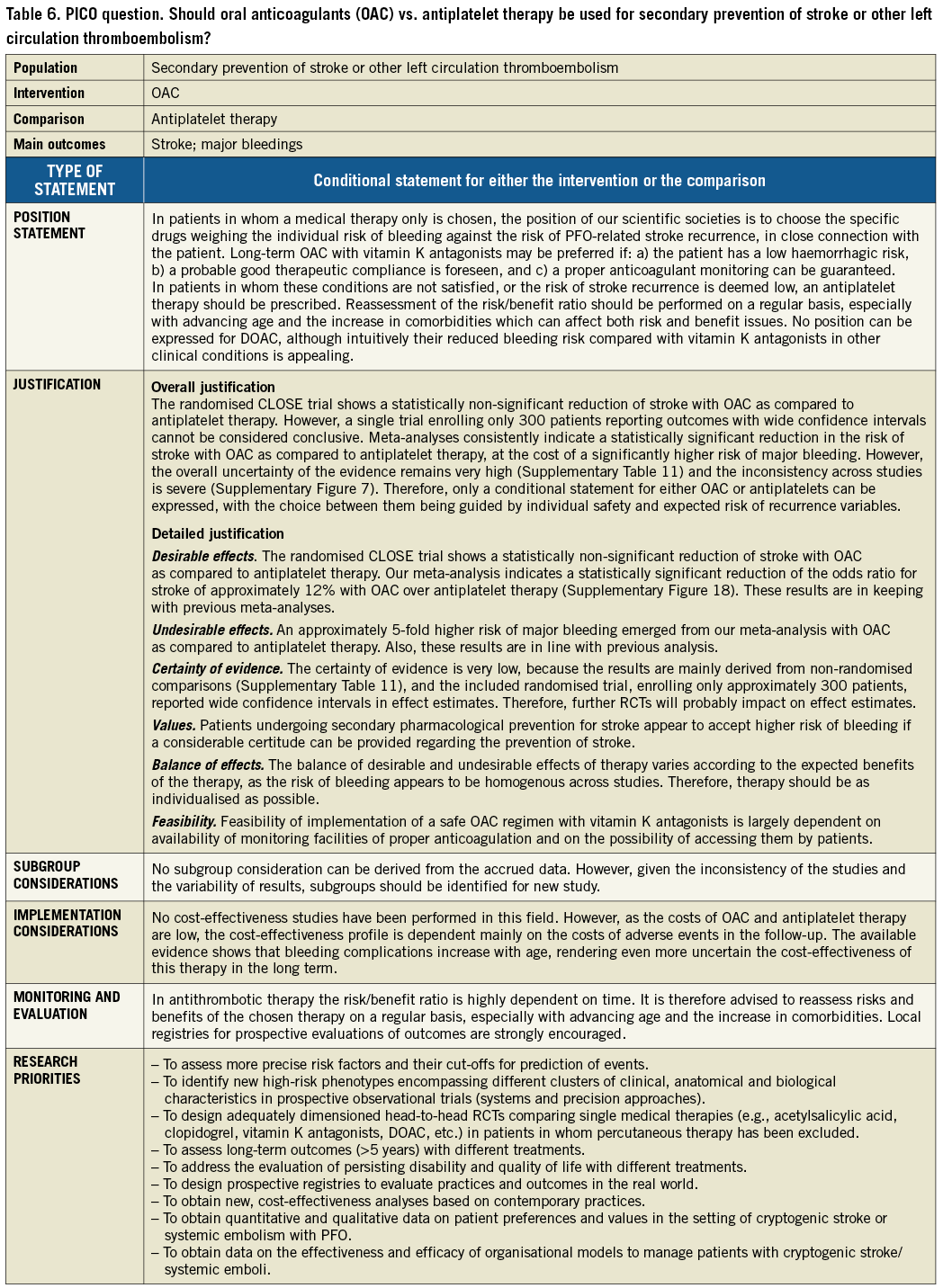
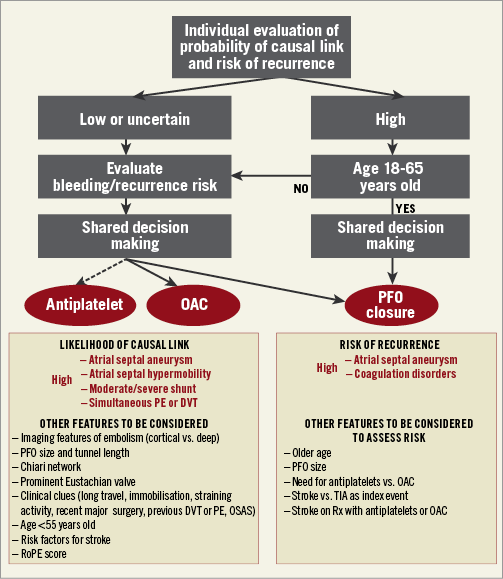
Figure 4. Treatment algorithm for secondary prevention of left circulation cryptogenic thromboembolism. DVT: deep vein thrombosis; OAC: oral anticoagulants; OSAS: obstructive sleep apnoea syndrome; PE: pulmonary embolism; Rx: therapy; TIA: transient ischaemic attack
EFFICACY AND SAFETY OF MEDICAL THERAPY
A variety of medical treatments has been used, based upon data from secondary prevention studies for stroke in general and from studies on cryptogenic stroke in particular. No adequately dimensioned RCT has yet been published that has assessed the effectiveness of individual drugs specifically in PFO-associated cerebrovascular accidents.
Trials were almost exclusively observational with only one adequately dimensioned RCT comparing oral anticoagulants (OAC) and antiplatelet agents. One meta-analysis of RCTs showed a recurrent stroke rate of 1.27 events per 100 patient-years with drugs only74. In our meta-analysis of the RCTs, the incidence of recurrent stroke on medical therapy was 4.6% after 3.8 years of follow-up (Supplementary Figure 4A, Supplementary Figure 4B, Supplementary Appendix 3), whereas in a meta-analysis of observational trials the recurrence rate was 5% per year113.
Despite a severe heterogeneity of results, the most recent meta-analysis including the randomised study is consistent with previous meta-analyses of observational studies113-116, suggesting superiority of OAC over antiplatelet agents in the prevention of stroke (Supplementary Figure 7, Supplementary Appendix 3). Although the overall quality of the evidence in this meta-analysis was estimated to be very low (Supplementary Table 11), the superiority of OAC vs. antiplatelet agents was also evident when considering studies with multivariate adjustment only (Supplementary Figure 7). No data are available on persisting disability and quality of life.
Reports on safety have often been incomplete or have yielded inconsistent results. In a meta-analysis of observational studies, 1.1% of patients receiving medical therapy experienced a bleeding complication113. This surprisingly low proportion of bleeding episodes can be explained by the young age of the patients and the short follow-up and, thus, must be interpreted with caution because most of these patients will undergo a lifelong medical therapy with an incremental risk of bleeding with age. Indeed, in our meta-analyses on PFO patients, an odds ratio (OR) of 4.57 was found for major bleeding with OAC relative to antiplatelet drugs (Supplementary Figure 8). A previous meta-analysis considering secondary prevention of stroke in general revealed that the potential benefit of OAC might be outweighed by the risk of both intracranial haemorrhage (OR 2.54) and major extracranial haemorrhage (OR 3.43)117. In this respect, direct oral anticoagulants (DOAC) may alter the risk-benefit ratio118,119, although no data exist in these patients.
SAFETY AND EFFICACY PROFILE OF PFO CLOSURE
Percutaneous procedure
Primary technical success approaches 100%78,113 and complete closure is seen in 93-96% at one year122. The use of larger devices has a higher risk of residual shunts113,123-125; the AMPLATZER™ PFO Occluder (St. Jude Medical, St. Paul, MN, USA) may have lower residual shunt rates than other devices123,125-130. Individual randomised data show a relative risk reduction of up to 80% for recurrent strokes131,132. One meta-analysis of RCTs has shown the stroke recurrence rate to be 0.29 per 100 person-years74 (Supplementary Appendix 3). In our study-level meta-analysis of RCTs with an average 3.8 years of follow-up, the incidence of recurrent stroke was 2% in the closure arms, and the number needed to treat (NNT) with PFO closure to prevent one stroke overall was 37 (95% confidence interval [CI]: 26 to 68) (Supplementary Figure 4A), and 21 in patients with high-risk PFO features (95% CI: 16 to 61) (Supplementary Figure 5). Results on TIA and on death were neutral (Supplementary Figure 4C, Supplementary Figure 4D, respectively). An increase of the treatment effects over time can be expected28,133,134. No data are available on persisting disability and quality of life.
Complications are summarised in Supplementary Table 5. Procedural complications had a 2.6% incidence in RCTs74. The most frequent late complication is device thrombosis, which is seen in 1.0-2.0%135. Device embolism is a serious event and occurs at a rate of 0.9-1.3%135,136. Atrial wall erosions are serious events that have been reported anecdotally. The risk of long-term mortality or the need for cardiac surgery is less than one in 1,000. Minor complications occur only in 1.0-1.7%.
The most frequent undesirable event following transcatheter percutaneous closure is AF in RCTs and observational trials28,78,106-111,113. In a meta-analysis of RCTs, a 4.6% incidence was reported after 3.8 years of follow-up74. In our meta-analysis, for incident AF, the overall number needed to harm (NNH) was 25 (Supplementary Figure 9A), whereas beyond 45 days there was no increased risk for AF with PFO closure (Supplementary Figure 9B, Supplementary Figure 9C). The incidence of these events was lowest with the AMPLATZER PFO Occluder (Supplementary Figure 10). Interestingly, a statistically significant reduction of AF prevalence after percutaneous closure of PFO was also shown in other studies, suggesting some antiarrhythmic effect of the procedure137.
Management after percutaneous closure
No data on best management strategies after PFO closure are available. Position statements are summarised in Table 7.
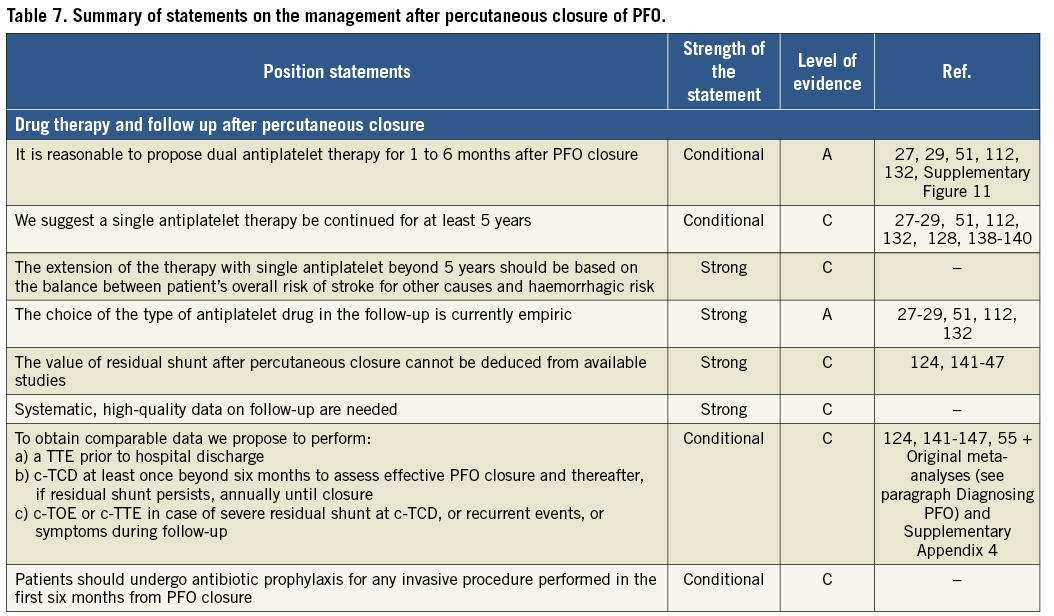
Drug treatments
To decide on post-procedural therapy one should consider that: a) endocardialisation of the device can continue up to five years post implantation128,138-140; b) one of the most frequent complications after closure is device thrombosis; and c) premature discontinuation of therapy may cause minor cerebrovascular events after PFO closure, as suggested by a marked trend towards association between duration of dual antiplatelet therapy after PFO closure and the incidence of TIA in our study-level meta-regression analysis (Supplementary Figure 11).
It is reasonable to decide on the post-procedural therapy according to the strategies used in RCTs. Overall, 5/6 RCTs prescribed or recommended a dual antiplatelet therapy in the first one to six months after closure, continuing with a single drug beyond two years in 3/4 RCTs that had a longer follow-up after that limit. In all positive trials, an antiplatelet therapy was prolonged for the entire duration of the study in the majority of patients (in 2/4 studies it was prescribed for five years). In one negative trial, only 50% and 41% of patients were still taking an antiplatelet therapy after one and five years, respectively132.
Delayed complications
Supplementary Table 5 displays the main tools available to detect complications. At present, no relationship between PFO patency after closure and the incidence of recurrence has been found (Supplementary Table 6)124,141-147, but studies were small, often plagued by partially incomplete follow-up, and problematic regarding shunt detection accuracy139. Also, a persistent shunt after closure may reveal other sources of paradoxical embolism which were missed during the diagnostic phase148.
No high-quality data are available to guide the optimal management of a residual moderate-to-severe PFO patency. The literature on acute and long-term results after repeat device implantation for a residual shunt is scarce, but retrospective evaluations are encouraging149-156.
Empirically, antibiotic prophylaxis against endocarditis before an invasive procedure or surgical intervention should also be considered routinely in all cases within the first six months after the implantation and, probably, beyond six months in patients with a residual shunt.
Surgical closure of PFO
There are no current indications for surgical closure of a PFO as first-line treatment. Closure of incidental PFOs is usually undertaken during valvular surgery or in the rare cases when surgery is indicated for other conditions in which the PFO plays a role, such as a straddling thrombus in the PFO, or seldom when complications of percutaneous closure occur which cannot be managed by percutaneous means.
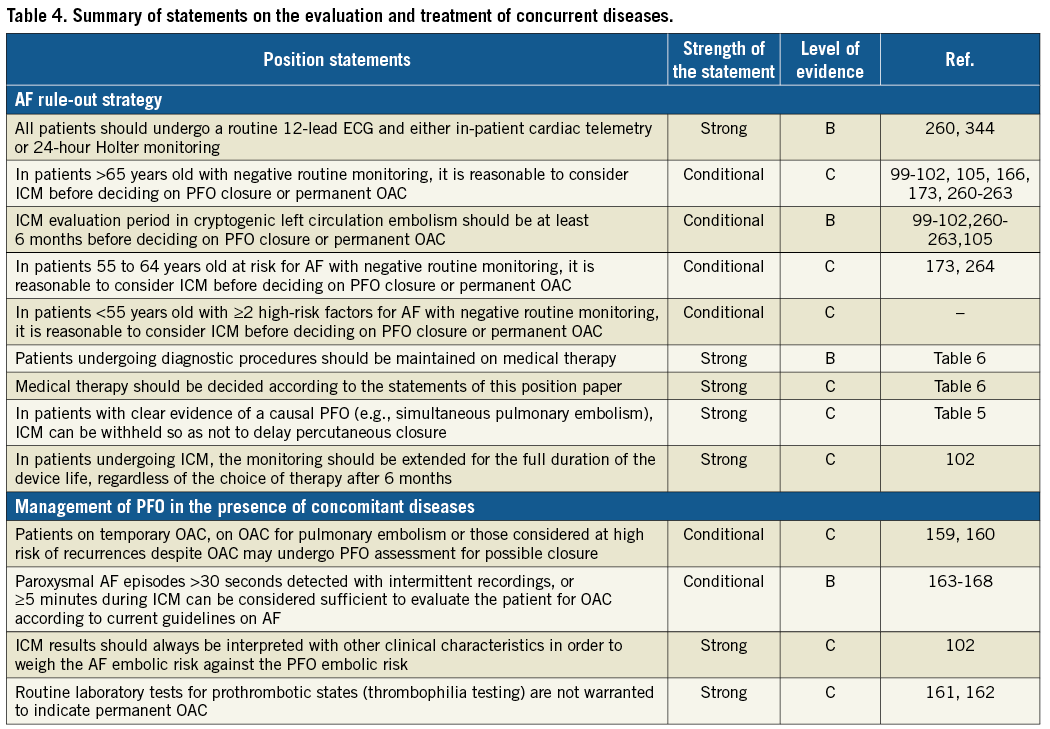
Management in the presence of concomitant diseases
Position statements are summarised in Table 4.
In the setting of hypercoagulability, deep vein thrombosis and/or pulmonary embolism159, PFO closure may be considered when there is the need for only temporary OAC or a high risk of recurrence despite permanent OAC, particularly in pulmonary embolism, where PFO was reported to be an independent predictor of new brain lesions in the follow-up, despite optimal OAC160.
Routine laboratory tests for prothrombotic states (thrombophilia testing) are not generally warranted to guide the need for permanent OAC161,162.
Although no study has assessed this issue as yet, it is reasonable that excluding patients with AF from PFO closure and treating them with permanent OAC should translate into an increased effectiveness of secondary prevention of left circulation thromboembolism. However, as in the CRYSTAL-AF study, a higher incidence of AF at ICM did not translate into a higher stroke incidence102; the presence of short bursts of AF on an ICM may carry a lower pathogenic value than a high-risk PFO. Therefore, the burden of AF should be weighed against the burden of PFO by considering other clinical characteristics to decide for or against PFO closure. For patients with paroxysmal AF, there is uncertainty regarding the duration of arrhythmic episodes which increases the risk of embolism. According to the HRS/EHRA/ECAS expert consensus statement on AF ablation, AF episodes ≥30 seconds constitute clinically significant AF163. During prolonged monitoring, episodes of AF ≥5 minutes have a predictive value for embolism164-168. These criteria should be combined with a thromboembolic score to evaluate the need for OAC169.
Conclusions
The management of patients with cryptogenic left circulation thromboembolism and PFO has been controversial, giving rise to heterogeneous strategies across different local realms in Europe. Based on the best available evidence, we were able to reach, in this interdisciplinary position paper, a consensus among eight European scientific societies on key diagnostic, therapeutic and research issues, from the index event to follow-up. It was possible to express strict position statements based on randomised trials for some therapeutic aspects, whereas other aspects were often based on limited and non-randomised data. This position paper provides the first largely shared approach for a rational PFO management based on the best available evidence. This may help physicians to offer coherent strategies throughout Europe and focus the research on high-priority subjects.
Guest Editor
This paper was guest edited by David R. Holmes, MD, MACC; Department of Cardiology, Mayo Clinic, Rochester, MN, USA.
Acknowledgements
We are indebted to Marielle De La Torre, Jenny Pena, Laurence Fani and the entire Specialty Centre Division of the European Association of Percutaneous Cardiovascular Interventions for their invaluable and highly professional support throughout the phases of development of this position paper.
Conflict of interest statement
H. Sievert reports institutional fees from Carag and Lifetech, outside the submitted work. B. Dalvi reports being a consultant to Abbott. B. Meier declares a conflict of interest in the form of speaker fees received from Abbott. M. Chessa reports personal fees from Abbott and Occlutech. D. Toni reports personal fees from Boehringer Ingelheim, Bayer, Pfizer Bristol-Myers Squibb, Daiichi Sankyo, and Medtronic. P. Scacciatella reports personal fees from Abbott Medical and Gore Medical. J. Thomson reports being a proctor and consultant for Abbott Medical and Gore Medical. D. Hildick-Smith reports having consultancy/advocacy for Abbott, Gore and Occlutech. D. Sibbing reports personal fees outside the submitted work from Roche Diagnostics, Daiichi Sankyo, Eli Lilly, Bayer Healthcare, Sanofi, Pfizer and AstraZeneca. S. Kasner reports personal fees from Bristol-Myers Squibb, Boehringer Ingelheim, Medtronic and AbbVie, grants from W.L. Gore, and grants and personal fees from Janssen and Bayer, outside the submitted work. G. Biondi-Zoccai reports having been a consultant for Abbott Vascular and Bayer. J. Carroll reports personal fees from AGA Medical, St. Jude Medical, and Abbott. The Chairman of the task force and all the other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
The references can be found in the Supplementary data document.
Supplementary data
Supplementary Appendix 1. Detailed methods.
Supplementary Appendix 2. Statistical methods.
Supplementary Appendix 3. Systematic review of evidence, assessment of its quality and meta-analyses.
Supplementary Appendix 4. Detailed evaluation of specific issues.
Supplementary Appendix 5. GRADE evaluation of evidences for PICO questions.
Supplementary Figure 1. Comparison of the rate of PFO detection in studies using TOE or autopsy.
Supplementary Figure 2. Meta-analysis of diagnostic accuracy studies comparing c-TCD vs. c-TOE.
Supplementary Figure 3. Meta-analysis of diagnostic accuracy studies comparing c-TTE vs. c-TOE.
Supplementary Figure 4. Outcomes in patients undergoing percutaneous closure or medical therapy.
Supplementary Figure 5. Subgroup analysis according to PFO features.
Supplementary Figure 6. PRISMA flow chart for the review of studies on the predictors of stroke in patients with cryptogenic stroke and a PFO.
Supplementary Figure 7. Forest plot for the risk of stroke recurrence in studies comparing OAC with antiplatelet therapy for cryptogenic solid systemic embolism.
Supplementary Figure 8. Forest plot for the risk of major bleedings in studies comparing OAC with antiplatelet therapy for cryptogenic cerebral or systemic embolism.
Supplementary Figure 9. Risk of atrial fibrillation in studies comparing PFO closure with medical therapy for cryptogenic cerebral or systemic embolism.
Supplementary Figure 10. Forest plot for the risk of atrial fibrillation according to the type of device used for PFO closure in studies comparing PFO closure with medical therapy for cryptogenic cerebral or systemic embolism.
Supplementary Figure 11. L’Abbé plots for the risk of TIA and stroke after PFO closure according to the length of dual antiplatelet therapy.
Supplementary Figure 12. Risk of stroke recurrence in patients undergoing OAC or antiplatelet therapy in studies comparing PFO closure with medical therapy for cryptogenic cerebral or systemic embolism.
Supplementary Figure 13. Risk of stroke recurrence according to the type of index event in studies comparing PFO closure with medical therapy for cryptogenic cerebral or systemic embolism.
Supplementary Figure 14. Risk of stroke recurrence according to the type of device used for PFO closure in studies comparing PFO closure with medical therapy for cryptogenic cerebral or systemic embolism.
Supplementary Figure 15. PRISMA flow chart for the review of studies on the RoPE score.
Supplementary Figure 16. PRISMA flow chart.
Supplementary Figure 17. PRISMA flow chart for the review of RCTs comparing PFO closure with medical therapy.
Supplementary Figure 18. PRISMA flow chart for the review of studies comparing OAC with antiplatelet therapy for secondary prevention of stroke, TIA or systemic solid embolism in patients with previous cryptogenic left embolism.
Supplementary Figure 19. PRISMA flow chart for the review of studies investigating the accuracy of PFO diagnostic tests.
Supplementary Figure 20. PRISMA flow chart for the review of studies investigating the accuracy of PFO diagnostic tests.
Supplementary Figure 21. Simplified scheme of the interacting network of processes underlying clinical manifestations associated with PFO.
Supplementary Table 1. PFO diagnostic methods.
Supplementary Table 2. Qualitative evaluation of diagnostic studies.
Supplementary Table 3. Qualitative evaluation of the studies on RoPE score.
Supplementary Table 4. Predictors of cryptogenic stroke recurrence in the presence of a PFO.
Supplementary Table 5. Complications of percutaneous closure.
Supplementary Table 6. Prognosis of patients with PFO patency.
Supplementary Table 7. Summary of meta-analyses on closure vs. medical therapy trials.
Supplementary Table 8. Observational studies comparing PFO closure with medical therapy.
Supplementary Table 9. RCTs comparing percutaneous closure and medical therapy.
Supplementary Table 10. Qualitative and quantitative assessment of the evidence on the comparison between PFO closure versus medical therapies.
Supplementary Table 11. Qualitative assessment of the evidence on the comparison between antiplatelet versus OAC therapies.
Supplementary Table 12. Quality of evidence grades.
To read the full content of this article, please download the PDF.

