Abstract
Aims: To evaluate the very long-term risk of recurrent thromboembolic events in patients treated by percutaneous PFO closure.
Methods and results: Between 1998 and 2008, a total of 232 consecutive patients with PFO and a high suspicion of paradoxical embolism were treated by percutaneous closure. The following major events were observed during hospitalisation: implantation failure (one patient) and appearance of an acute left-sided device thrombus requiring surgery (one patient). The primary endpoint of the study was a recurrent embolic event beyond at least five years’ follow-up. During a mean follow-up of 7.6±2.4 years, this event occurred in five patients, representing a 0.28% annual/patient risk. Other major complications during follow-up were the following: late thrombus formation on the device (two patients) and transient atrial fibrillation (15 patients). Three patients died during follow-up from cardiovascular causes considered not related to the index procedure. The PFO was judged closed on follow-up echocardiography in 92.3% of patients.
Conclusions: Long-term follow-up following percutaneous PFO closure for presumed paradoxical embolism reveals very low recurrence rates. This observation should be put in perspective with recent published randomised trials comparing percutaneous closure and medical therapy.
Abbreviations
ASA: atrial septal aneurysm
DAPT: dual antiplatelet therapy
LMWH: low-molecular-weight heparin
PFO: patent foramen ovale
TIA: transient ischaemic attack
TOE: transoesophageal echocardiography
Introduction
A patent foramen ovale (PFO) is a remnant of the foetal circulation allowing direct access of the pulmonary to the systemic circulation. At birth, changes in intrathoracic pressure will induce spontaneous PFO closure in the vast majority of the general population. Nevertheless, autopsy series have revealed the persistence of a PFO in up to 27% of patients1. The potential association between a PFO and paradoxical embolism has been known for decades and has therefore been associated with certain pathologies2. Conceptually, thrombus, desaturated blood, gas and molecular triggers may migrate through the PFO and be related to these clinical entities. Cryptogenic stroke, platypnoea-orthodeoxia syndrome, unexplained diving accidents and migraine with aura have all been correlated with a PFO2. Exceptional case studies have been published showing direct evidence of thrombus impacting on a PFO causing stroke3. These observations have triggered interventions to prevent recurrence of embolic events. Historically, surgical closure was performed in the 1990s yielding variable outcomes4,5. In 1992, percutaneous closure was attempted, at first using a clamshell device6. Despite the lack of evidence, percutaneous closure was increasingly performed and reported in several observational studies7. These observations have promoted the initiation of several randomised trials comparing medical therapy and percutaneous closure, in particular in the setting of cryptogenic stroke8-10. These trials turned out to be negative compared to medical therapy, and only a post hoc meta-analysis of the data demonstrated a lower recurrence of stroke and TIA with device therapy11. Interestingly, recurrence rates were much lower than projected, explaining in part the negative results of each trial separately.
At both institutions including patients in the current study, percutaneous PFO closure has been performed in close collaboration with neurologists since 1998. An identical methodical clinical work-up for cryptogenic stroke has been applied since then, implementing stringent inclusion criteria for percutaneous closure. The hypothesis of the current investigation was that long-term clinical follow-up of our study population should demonstrate comparable recurrence rates to recently reported randomised trials.
Methods
PATIENTS
Percutaneous PFO closure has been performed at our institution since 1998. Clinical work-up in young (18-55 years old) cryptogenic stroke patients included MRI, MR or CT angiography of cervical and cerebral arteries, cardiac rhythm monitoring and clinical three-month follow-up in order to identify potential causes of stroke. Standard transthoracic echocardiography with microbubble testing using agitated saline to check for the presence of a PFO was systematically performed. The presence of a PFO was highly suspected if microbubbles crossed within the first four cardiac cycles after injection. The diagnosis was confirmed by transoesophageal echocardiography enabling a more precise analysis of the anatomy, paying particular attention to the presence of an atrial septal aneurysm (ASA: defined as an excursion of >15 mm on M-mode echocardiography). Echocardiographic work-up included basic image acquisition at rest as well as during the Valsalva manoeuvre. The degree of shunting was classified as described, as follows: grade 1 (weak, <10 bubbles), grade 2 (moderate, 10-30 bubbles), and grade 3 (important, >30 bubbles). Patients were systematically screened for the presence of a coagulation disorder: presence of factor V Leiden, antithrombin III, proteins C and S, and antiphospholipid and anticardiolipin antibodies.
As far as cryptogenic stroke is concerned, patients were proposed percutaneous closure in the presence of the following criteria: 1) age 18-55 years, 2) presence of a grade 3 shunt at rest or Valsalva, and/or presence of an ASA, 3) patients with a grade 2 shunt only considered for closure if they had experienced recurrent cryptogenic stroke. Patients were discussed at staff meetings between neurologists and cardiologists. Upon consensus agreement and according to these criteria, patients were referred to the cardiologist to provide detailed information about the procedure, its risks and potential advantages and the therapeutic alternatives. If the patient accepted the intervention and written informed consent was obtained, the intervention was planned.
After intervention, patients were followed up both by the cardiologist (microbubble test at three and six months, one and two years to check for closure of the PFO) and by the neurologist.
PROCEDURE AND DEVICES FOR CLOSURE
Between 1998 and 2003, procedures were realised under general anaesthesia and guided by transoesophageal echocardiography (TOE). Briefly, after final quantification and qualification of the shunt by injection of agitated saline through the right femoral vein sheath at rest and during forced Valsalva manoeuvre (with the help of the anaesthesiologist), the PFO was crossed with a soft J-tipped 0.035 inch wire and 6 Fr multipurpose catheter. After positioning of the multipurpose catheter in the left upper pulmonary vein, wires were exchanged for a 260 cm 0.035 inch wire allowing positioning of the Mullins device delivery catheter. The following devices were used: PFO-Star (Cardia Inc., Eagan, MN, USA), STARFlex and BioSTAR (NMT Medical, Boston, MA, USA), and the Amplatzer PFO occluder (St. Jude Medical, St. Paul, MN, USA). Since 2003, as intracardiac echocardiography became available at our institution, procedures were performed under local anaesthesia with access from the left femoral vein to introduce the probe (AcuNav™; Biosense Webster Inc., Diamond Bar, CA, USA). Both echocardiography and fluoroscopy were used to guide device deployment. Patients received an intravenous dose of cefazolin (1 g just prior to and 1 g after the procedure) and were all treated with aspirin (100 mg) and clopidogrel (75 mg) prior to intervention from 2001. In general, clopidogrel was stopped at three months. Aspirin therapy was only maintained if a significant shunt persisted beyond six months. Between 1998 and 2001, patients received a combination of aspirin and warfarin for a duration of six months (covered by injections of low-molecular-weight heparin [LMWH] up to efficacy of oral anticoagulation from the third patient treated on). Those patients with a coagulation disorder on warfarin were temporarily withdrawn from oral anticoagulation and bridged by LMWH for the time of the intervention. Aspirin was added to their treatment for a period of three months. Patients were also instructed to follow endocarditis prophylaxis for at least one year.
STUDY ENDPOINTS
The primary study endpoint was recurrence of stroke or TIA during a follow-up of at least five years during a 10-year study period stretching from 1998 to 2008. Secondary endpoints were acute and long-term procedural success, defined respectively as a procedure without any complication and complete PFO closure on microbubble testing at follow-up. Any other complication was also traced as a secondary endpoint.
DATA COLLECTION AND STATISTICAL ANALYSIS
Patients were prospectively entered into dedicated databases by both the implanting cardiologists and the neurologists and followed up on a regular basis. For study purposes, both databases were cross-matched for any inconsistencies. If patients were referred from elsewhere, contact was taken up with the referring cardiologist or exceptionally, if needed, directly with the patient. Only basic descriptive statistics were used. Values are expressed as absolute numbers or percentages. Mean values are indicated with standard deviations. The primary endpoint is expressed as percentage per patient follow-up years. The hospital ethics committee had approved the study protocol.
Results
PATIENT AND PROCEDURAL OUTCOMES
A total of 232 patients have been treated over the 10-year study period. Baseline demographics are presented in Table 1. As expected, with a mean age of 46 years, patients were rather young and presented a low incidence of general risk factors for atherosclerosis. A few patients were older than 55 years. These were all patients with desaturation syndromes, with the oldest patient being 77 years. The indications for intervention are listed in Table 2. The main indication for closure (90.5%) was cryptogenic stroke or TIA. Uncommon indications were: recurrent unexplained diving accidents in professional divers (six patients) and closure prior to liver transplantation (one patient). This table also shows that 69.4% of patients presented with an ASA, reflecting the presence of a large PFO in the majority of patients. Procedures were guided by TOE in the first 91 patients (39.2%) or intracardiac echocardiography in the remaining 141 patients (60.8%). Figure 1 depicts the proportions of the different devices used. The PFO-Star was used initially until 2001 in 23 patients (10%). From that year on, the STARFlex occluder was predominantly implanted (145 patients, 62.5%) until 2007 when the BioSTAR (53 patients, 22.8%) became available. Sporadically, the Amplatzer PFO occluder (11 patients, 4.7%) was used. A typical example of a PFO procedure is illustrated in Figure 2. An intracardiac echo probe is positioned in the right atrium and the typical double umbrella structure of a closure device before and after release is shown.
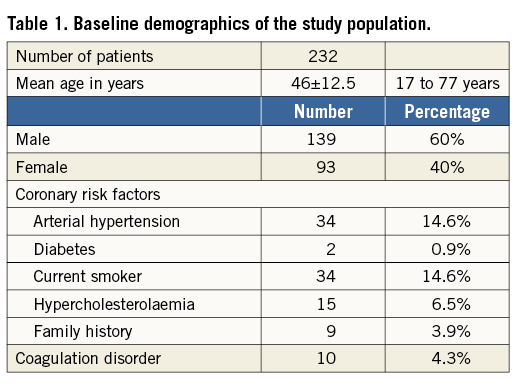
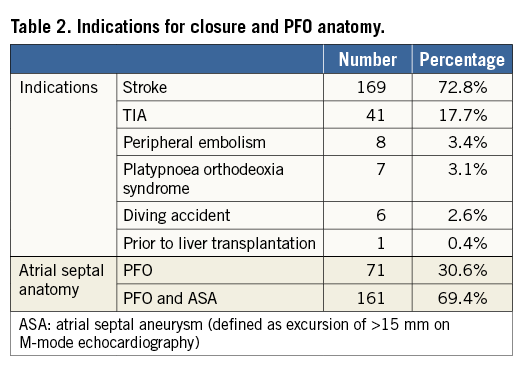
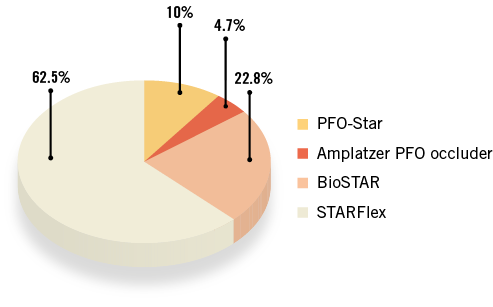
Figure 1. Overview of the different devices used during the study period.
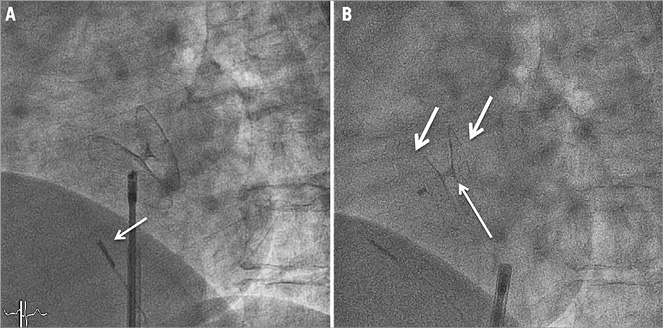
Figure 2. Illustration of a typical PFO closure intervention. A) Fluoroscopic view of a closure device during its deployment. An intracardiac echo probe (arrow) is positioned anterior to the device that is still attached to its delivery system. B) Fluoroscopic view after release. The double umbrella structure of the implant (right and left-sided parts indicated by arrows) can be appreciated. The thin arrow indicates the position of the PFO channel.
PRIMARY STUDY ENDPOINTS
During a mean follow-up of 7.6±2.4 years, a total of five patients experienced recurrent embolic events, all of them ischaemic strokes. These events occurred between seven months and five years after intervention. Only one of these patients had a persistent large shunt through the implant and therefore a second device was successfully added to close the PFO. Considering the 218 patients only treated for suspected thromboembolism, the calculated yearly incidence of stroke/TIA is therefore 0.28% patient/year.
SECONDARY STUDY ENDPOINTS
Implantation was successful in all but one patient. A STARFlex 33 mm device had to be withdrawn because of an unstable position in a very large PFO, and the patient refused a second attempt with a different device. Therefore, the acute procedural success rate was 99.6%.
Transient atrial fibrillation in the acute phase was noted in two patients. One patient developed an important haematoma at the puncture site (treated conservatively), and eight patients experienced a transient coronary air embolism. One patient developed a transient brachial plexus paralysis in the setting of general anaesthesia. The most severe acute complication occurred in the second study patient closed during the early experience in 1998. This patient developed an acute left ventricular thrombus on a first-generation PFO-Star device that was discovered accidentally by echocardiography the day after intervention. At that time, warfarin was used immediately after closure, and treatment was not accompanied by LMWH during the initial period. The decision was taken to remove the implant and close the PFO surgically. Warned by this observation, patients were bridged with LMWH till effective oral anticoagulation from that moment on until 2001, when it was empirically decided to switch to dual antiplatelet therapy (DAPT). Therefore, the rate of major periprocedural complications was 1.3% (implantation failure, brachial plexus and acute device thrombosis).
Figure 3 depicts the regression of the shunt on microbubble testing before and after intervention. The PFO was considered closed in 93.2% of patients (87.8% with complete closure and 5.4% with a mild grade 1 shunt).
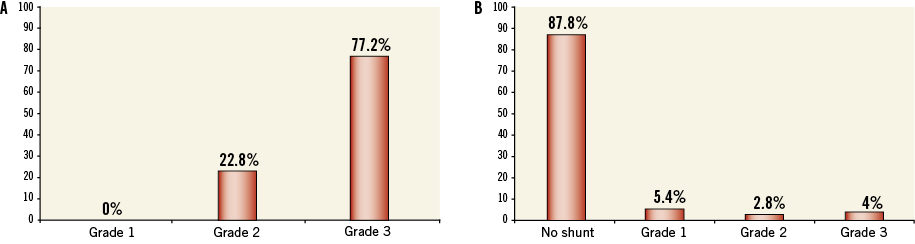
Figure 3. Assessment of PFO closure prior to and after intervention. A) Degree of shunting as observed during microbubble testing before intervention. B)Degree of shunting after closure (values in percentages).
During the long-term follow-up, two late device thromboses were observed. One patient developed a right-sided device thrombus on a PFO-Star device at six months that was treated successfully by prolonged oral anticoagulation. In the other patient, a left-sided thrombus was discovered two years after intervention on a STARFlex device. As no regression appeared despite prolonged oral anticoagulation, the device was removed surgically and the intra-atrial septum was repaired. Another patient (first-generation PFO-Star) developed an asymptomatic fistula between the right atrium and the aorta that closed spontaneously six months after cessation of oral anticoagulation. Fifteen patients (6.5%) experienced transient, short episodes of atrial fibrillation within the first months after intervention. None required electrical cardioversion. Eleven patients were temporarily treated by warfarin and antiarrhythmic drugs.
Three patients died during follow-up, all from cardiovascular causes. These events (myocardial infarction [n=2], aortic dissection [n=1]) occurred between four and eight years after intervention and were considered not related to the PFO closure.
Discussion
The present study sought to investigate the very long-term risk for recurrent thromboembolic events after PFO closure for presumed paradoxical embolism. The main findings of this observational study conducted over a 15-year time period in 232 patients were that, taking into account progress in technique and devices during the early phase, this intervention is safe and effective in reducing recurrent events with a calculated annual/patient risk of 0.28%, a number that is lower than usually reported.
EFFICACY OF PERCUTANEOUS PFO CLOSURE
Numerous studies have investigated the efficacy of PFO closure in the prevention of thromboembolic events. Historically, several observational studies have reported rather short-term outcomes, occasionally matched with a group of patients treated medically7,12. More recently, between 2012 and 2013, three randomised controlled trials comparing medical and interventional therapy have been published. All trials (CLOSURE 1, PC and RESPECT) failed to reach the composite primary endpoint of all-cause mortality and recurrent stroke and/or TIA8-10. A full description and analysis of trial design and results lie beyond the scope of this paper. Briefly, the annual recurrence rate in the closure arms was higher in CLOSURE 1 (2.9% stroke and TIA in the per protocol population), and rather low with 0.8% in the PC-Trial (stroke and TIA in closed patients) and 0.4% in RESPECT (stroke in the per protocol population). The publication of these trials has initiated a few meta-analyses yielding different conclusions, at variance with the trend towards a significant reduction of recurrent neurological events11,13-15. The general belief is that the relatively short follow-up was the main reason for the unanticipated low event rates. This low PFO-related recurrence risk is also one conclusion of the RoPE (Risk of Paradoxical Embolism) study group that has shown an annual recurrence rate of about 1% in non-closed patients even in the presence of a high likelihood of an initial PFO-related event16.
Only Wahl et al have investigated the long-term outcome after PFO closure (n=150) and compared this to a matched control group of medically treated patients (n=158) from the Bern stroke registry12. They evaluated an endpoint identical to the one in the present study at a median follow-up of 10 years, and observed a relative reduction of 43% with device therapy. This number is quite comparable to the results of one of the previously cited meta-analyses. The accumulated patient-years were 1,582 (closure) and 1,685 (medical therapy). The calculated annual/patient risks were therefore 0.69% for device versus 1.31% for medical therapy. With 1,657 patient-years in the present study and an annual/patient risk of 0.28%, our results compare favourably to the analysis of Wahl et al. The larger patient-years value is explained by the difference in patients treated by PFO closure in the present study (218 versus 150) despite a slightly shorter follow-up period. Of note, only 24.7% of patients treated by closure by Wahl et al presented an ASA compared to 69.4% in the present study, reflecting, as mentioned before, more stringent criteria for closure, in particular the presence of a “larger PFO”.
Very recently, a randomised comparison of three different closure devices for PFO closure has been published by Hornung et al17. This study demonstrated different clinical outcomes depending on the device used. However, indications for closure were wider than commonly “accepted” including patients with migraine. Even if this trial does not have any direct relation with the current study, it adds further confusion to the field.
SAFETY OF PERCUTANEOUS PFO CLOSURE
As mentioned previously, the natural history of patients with suspected thromboembolism and PFO is characterised by low recurrence rates. Therefore, safety of percutaneous PFO closure is crucial, as this implies the implantation of a permanent device in young patients. In the present study, only a few complications were observed during the in-hospital phase, events that can all be attributed to the learning phase. The second patient of the study cohort, treated in 1998, developed a left-sided thrombus and was treated surgically. It is well known that warfarin during its initiation phase may exert a procoagulant effect if not covered simultaneously by LMWH. On the other hand, the implant used was a first-generation PFO-Star device, characterised by more abundant Ivalon tissue on its left side. From that moment on, all patients were covered by LMWH bridging. From 2001, on an empirical basis and in analogy with “coronary stenting practice”, patients were treated with DAPT, a practice that was initiated by most centres performing PFO closure at that time.
Closure was unsuccessful in only one patient presenting with a very large PFO. This complication may be attributed to the learning curve with STARFlex, a device the manipulation of which was known to be more complicated. Finally, one patient experienced a transient brachial plexus paralysis in the setting of general anaesthesia.
These in-hospital complications were all encountered during the first years. At that time, PFO closure was not common practice. Since then, technical progress, improved skills, abandonment of general anaesthesia and introduction of DAPT have virtually eliminated the early major hazards.
During follow-up, two “late thromboses” on the implant were observed, each time on a STARFlex device. Neither of the patients had a coagulation disorder. Krumsdorf et al have reported higher thrombosis rates with STARFlex (5.7%) and PFO-Star devices (6.6%) than with other devices11. This study, however, was retrospective and hampered by confounding factors, such as the presence of previously unknown coagulation disorders and/or atrial fibrillation. In our series, the incidence of thrombosis (0.9%) on STARFlex compares favourably to the series of Krumsdorf but is definitely still too high18. In 2007, BioSTAR became available at our institution, and since then early or late thrombosis has no longer been observed. This device was further preferentially used beyond 2008 until NMT, the manufacturer of the device, went bankrupt in 2011. Since 2011, the Amplatzer septal occluder and the Figulla Flex occluder (Occlutech, Helsingborg, Sweden) have been used, and no adverse event has been noted since then.
Since the first percutaneous PFO closure in 1992 and other preliminary experiences initiated in the late 1990s, device improvements have been realised, new devices have become available and others are no longer available. Taking into account more recent studies and in particular the recently published randomised trials, percutaneous closure appears to be a very safe technique.
Limitations
Although prospective in design, patients had to be contacted occasionally to update missing information. Over this long study period devices and pharmacotherapy have undergone substantial changes. This is a single-arm observation without a matched or randomised control arm. Furthermore, with 232 patients included, the study population remains relatively small.
Conclusions
This long-term follow-up study of patients treated by percutaneous PFO closure for secondary prevention of recurrent thromboembolic events reveals one of the lowest annual/patient risks ever reported. The current observation should be further confirmed by additional randomised trials in the field, which are currently enrolling study patients.
Conflict of interest statement
E. Eeckhout and A. Delabays both act as proctors for St. Jude Medical. P. Michel has received speaker fees from St. Jude Medical and is a member of the data safety and monitoring board of CLOSE. The other authors have no conflicts of interest to declare.

