Abstract
Aims: To summarise our experiences of device closure of residual shunt after transcatheter closure of patent foramen ovale (PFO).
Methods and results: Since October 1997 implantation of a second closure device was attempted in 40 patients with a moderate or large residual shunt after implantation of a PFO closure device. The mean age of the patients was 51 years. Implantation of a second closure device was technically successful in 39/40 patients (98 %). The following devices were implanted: Premere™ (n=20), Amplatzer® PFO (n=13), STARFlex® (n=4), Helex™ (n=1), Angelwings (n=1). During a mean follow-up of 36±29 months complete closure was achieved in 27 patients (69 %). The remaining shunt was small in nine patients, moderate in one and large in two. One patient with a moderate and one patient with a large residual shunt received a third device. The third patient was sent to surgery. One patient died 21 days after implantation of a third closure device due to acute pericardial tamponade. No other complications occurred.
Conclusions: Transcatheter implantation of a second closure device after PFO closure is feasible. Complete closure can be achieved in the majority of patients.
Abbreviation list
PFO: patent foramen ovale
TEE: transesophageal echo
Introduction
Patent foramen ovale (PFO) is a relatively common congenital condition that occurs in 27–30% of the population1. It may be involved in multiple disease processes including cryptogenic stroke and TIA2, peripheral3 and coronary embolism4, decompression sickness5 and migraine headache with aura6. The first case series reporting the successful transcatheter closure of PFO in 36 patients was published in 19927. Today, several newer devices are used to close PFOs. Complete closure rate with these devices ranges from 90% – 96%8-12. Nevertheless, the goal of all devices is to obtain a complete closure because there is a risk of recurrent cryptogenic stroke in patients with a residual shunt after transcatheter PFO closure13-15. Patients with residual shunt following percutaneous PFO closure can be treated with antiplatelet or anticoagulation drugs, surgery with device removal and closure of the PFO or percutaneous implantation of a second closure device. To date, only limited information is available about implantation of a second closure device. Here, we present our experience and long-term results in transcatheter closure of residual shunt after percutaneous PFO closure in 40 patients.
Methods
Between August 1994 and June 2008, 1,677 consecutive patients with a mean age of 50 years underwent percutaneous PFO closure in our centre. Most of the patients were treated for the indication of stroke, TIA, and peripheral embolism. Results of PFO closure in general, details regarding transcatheter device implantation, post-procedural medication and recommended follow-up examinations have been reported previously16-18. Five patients were lost to follow-up due to different reasons (relocation with no forwarding contact information, incomplete information, or refusal by the patient).
During follow-up ranging from one month to 165 months, mean 37±25 months complete closure was achieved in 1,565/1,672 patients (94%) which correlates to complete closure rates in previous studies8-12. In 107 patients a residual shunt remained. In eight of these patients residual shunting persisted due to newly discovered additional atrial septal defects. They were excluded from this analysis.
Table 1 gives an overview of the implanted devices and the number of devices in which a residual shunt remained during follow-up.
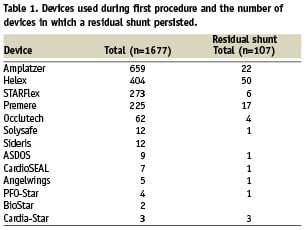
In all, TEE showed a large residual shunt in 13/99 patients (13%), a moderate residual shunt in 36/99 patients (36%) and a small residual shunt in 50/99 patients (51%) of the patients with incomplete closure of the PFO.
Residual shunting was graded as negative (no bubbles seen in the left heart), small (1-5 bubbles seen in the left heart), moderate (6-20 bubbles seen in the left heart), and large (>20 bubbles seen in the left heart)19. The bubbles were counted at rest and/or during Valsalva within three heart cycles after contrast opacification of the right atrium. Several contrast injections were performed and the highest amount of bubbles was noted.
All 49 patients with a moderate and large residual shunt on TEE were offered cardiac catheterisation and possible implantation of a second closure device.
Fourteen patients with a large or moderate residual shunt declined to be treated with a second closure device (one patient was sent to surgery, 13 patients were put on blood thinning therapy). Additionally, five patients in whom a first PFO closure device was implanted in an external centre were referred to our centre for implantation of a second closure device due to a moderate or large residual shunt.
Finally, a total of 40 patients with a moderate or large residual shunt agreed to be re-treated with a second closure device (Figure 1).
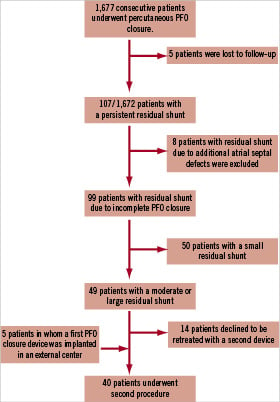
Figure 1. Flowchart summarising patients with a residual shunt after initial percutaneous PFO closure.
Recurrent events in patients with residual shunt
Two patients with a large residual shunt suffered from a recurrent thromboembolic event. In a 50 year old man embolism of the central retinal artery occurred five years after implantation of the first PFO closure device. A 60 year old man had a transient weakness of his left arm followed by a transient global amnesia four years after the initial procedure. In both patients no other potential stroke sources were found except for the large residual shunt and a second device was implanted successfully. No recurrent thromboembolic event occurred thereafter and residual shunting decreased to small in both.
Second procedure
Between October 1997 and January 2008 a total of 40 patients were referred to our centre for implantation of a second closure device.
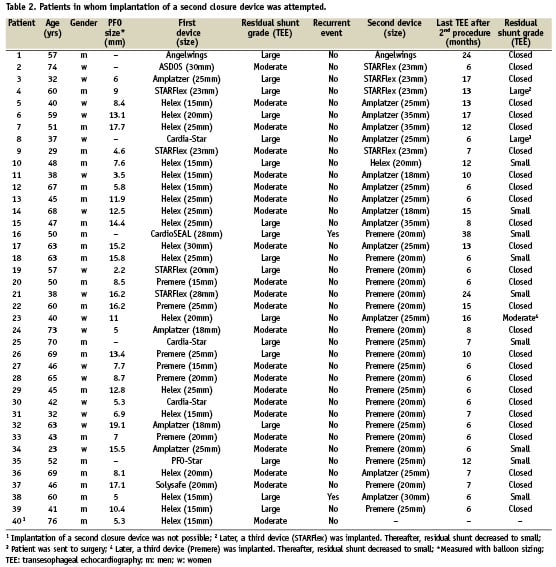
The mean age of the patients was 51±13 years (range 23–74 years). Based on the grading of residual shunting described above, 17 patients (42%) showed a large residual shunt and 23 patients (58%) a moderate one. Patient characteristics are summarised in Table 2.
Implantation of the second device was similar to the initial procedure and was performed under angiographic and echocardiographic guidance.
After right-heart-catheterisation with selective contrast injections in the area of the PFO, the residual gap of the PFO tunnel was crossed with a guidewire. Additionally, a TEE was performed during all procedures to evaluate the residual shunt, the anatomy of the atrial septum and to exclude an additional atrial septal defect. The type of device was chosen according to the atrial septal anatomy and previously implanted device. In 3/39 patients (8%) a device was implanted after puncture of the atrial septum. The last 29 patients received either an Amplatzer® or a Premere™ device during the last five years.
Before discharge an electrocardiogram and a transthoracic echocardiogram were performed. Due to the fact that there are no guidelines available regarding optimal postoperative antiplatelet strategies different antiplatelet therapies were initiated at our centre based on increased clinical experiences and devices. The first three patients who received a second closure device were discharged on a regimen of antiplatelet therapy with aspirin (100 mg) for six months. The following 31 patients were treated with aspirin (100 mg) and clopidogrel (75 mg) for six months and the remaining five patients were placed on aspirin (100 mg) for six months and clopidogrel (75 mg) for three months. All patients were advised to follow subacute bacterial and endocarditis prophylaxis for six months after the procedure. Follow-up examinations, including clinical investigation, electrocardiogram and transthoracic echocardiography, were recommended at one, six and twelve months after the initial procedure and annually thereafter. In addition, a transesophageal echocardiogram (TEE) was recommended at the one and six month follow-up. A questionnaire was sent every 12 months.
Results
Implantation of a second occluder was successful in 39 out of 40 patients (98%). In one patient it was not possible to cross the septum and a second device was not implanted. Fluoroscopy time of the procedure ranged from 1.9 min to 42 min (mean 8.6±8.6 min). All procedures were performed under local anaesthesia. There were no procedural complications.
A Premere™ (St. Jude Medical, St. Paul, MN, USA) occluder was implanted as a second closure device in 20 patients (51%), an Amplatzer® (AGA Medical Corp., Golden Valley, MN, USA) PFO occluder in 13 patients (33%), a STARFlex® (NMT Medical, Boston, MA, USA) occluder in four patients (13%), an Angelwings (Das Angel Wings, Microvena Corp., Vadnais, MN, USA) occluder in one patient (3%) and a Helex™ (W. L. Gore and Associates, Flagstaff, AZ, USA) occluder in one patient (3%). Only Amplatzer® and Premere™ occluders were used in the last 29 patients (74%).
An overview of devices used during the initial procedure and the corresponding devices used as second closure devices are summarised in Table 2.
During a mean follow-up of 36±29 months (range six months to 112 months) after implantation of a second device complete closure was achieved in 27 patients (69 %). In all 39 patients a TEE was performed after a minimum of six months after the second procedure (Table 2). More than a mild residual shunt remained in only three patients. In two of these patients a third closure device was implanted. The third patient declined to be treated with a third closure device and was sent to surgery. No recurrent thromboembolic events, no device embolisation, no new atrial fibrillation and no other complications occurred after implantation of a second closure device. However, one patient who received a third closure device died 21 days after the procedure due to an acute pericardial tamponade. Autopsy showed that one arm of the three implanted Starflex devices had perforated the aorta. At a follow-up examination 17 days after implantation the patient had no problems and the echocardiographic exam showed only a small residual shunt and no pericardial effusion or hemopericardium.
Discussion
One treatment option in patients with residual shunt after percutaneous PFO closure is percutaneous implantation of a second closure device. A small residual shunt, contrary to a moderate or large residual shunt, may likely be eliminated slowly over time with complete endothelialisation19,20 and several studies have shown that patients with a large degree of shunt across a patent foramen ovale, are at a significantly higher risk for subsequent adverse neurologic events21-23. Therefore, medical treatment in this patient population is reasonable. In our cohort, 50 patients showed a small residual shunt and were treated with blood thinning therapy. Fifteen patients with a moderate or large residual shunt did not receive a second closure device and were put on blood thinning therapy (13 patients declined to be re-treated, in one patient implantation of a second device was not possible, and one patient was sent to surgery). In eight patients additional atrial septal defects turned out to be the reason for residual shunting. Multiple atrial septal defects can be difficult to diagnose. Nevertheless, careful examination should be taken by echocardiography before and during the initial procedure to detect additional atrial septal defects and to examine whether all defects can be covered with one large device or more than one device is required.
Only little is known about the safety and efficacy of device closure of residual shunt after transcatheter PFO closure. In 2004, Schwerzmann et al presented the results of a small series of 10 patients who had undergone implantation of a second closure device due to residual shunt after percutaneous PFO closure24. Our experiences of 39 patients who received a second closure device show that implantation of a second device in patients with a moderate or large residual shunt is feasible. Over the time Amplatzer® and Premere™ devices have replaced all other devices for closure of residual shunt. Both devices have advantages which make them superior to other devices for closure of residual PFO gaps. The Amplatzer® occluder is easy to handle, fully repositionable, can be retrieved even after release from its delivery cable and is better suited for short-tunnel defects. This is very helpful in complex anatomy which is often the case in residual gaps of the PFO tunnel. Additionally, the Amplatzer® occluder can improve alignment of a previous implanted device by sandwiching it. Therefore, we used the Amplatzer® occluder especially in patients in whom a Helex™ device was implanted previously. The Helex™ occluder consists of a soft fabric and the alignment of the discs to the septum is sometimes not optimal which may result in residual shunting. Implantation of an Amplatzer® device can improve alignment of the Helex™ discs by sandwiching them.
We used transseptal puncture technique for implantation of a second closure device in three patients with a very long-tunnel residual defect because this foramen potentially can result in deformity of PFO devices. Later, when the Premere™ device became available transseptal puncture technique was not necessary anymore.
The Premere™ device was specifically designed to accommodate different PFO lengths and septal characteristics. Since a residual PFO gap often presents as a very long slit-shaped tunnel and the septum itself has a variable thickness, resulting in right and left atrial septal walls that are not parallel to one another, the specific design of the Premere™ device makes it well suited to be used as a second closure device.
We recommend using the Amplatzer® device as a second closure device in short-tunnel defects especially when a device with a soft fabric was implanted previously and the Premere™ device should be used in long-tunnel defects.
In our series complete closure was achieved in 27/39 patients (69%). Only three patients had more than a small shunt after implantation of a second device. In two of these patients a third closure device was implanted and only a small shunt remained. One patient declined to be treated with a third closure device and was sent to surgery which was uneventful. Neither recurrent thromboembolic events nor any complications occurred in patients who received a second closure device. One patient who received a third closure device died 21 days later.
In conclusion, implantation of a second closure device due to residual shunt after percutaneous PFO closure is feasible and safe when it is performed by an experienced interventionalist. Complete closure was achieved in the majority, but is lower than after transcatheter closure of a native PFO. Even implantation of a third device is feasible but seems to increase the risk of erosion.

