Abstract
Aims: The success rate in eliminating a right-left-shunting following percutaneous patent foramen ovale closure is estimated to be >90%. However up to 10% of patients may have residual shunting following initial closure. Little is known as to the optimum treatment strategy for these patients. We report four cases in which to redo patent foramen ovale closure was possible with a second device.
Methods and results: At our institution during 2008-2009, 101 patients underwent PFO closure: 81 patients (80%) underwent PFO closure for cerebrovascular events, 12 patients (12%) for migraine with aura, eight patients for systemic embolisation (8%), three patients (3%) for decompression illness and one patient underwent PFO closure for platypnea-orthodexia syndrome. Irrespective of the initial device, redo closure was technically feasible in all cases. All patients had at least a moderate residual shunt evident on echocardiographic evaluation at > 6-month follow-up. The patients in the current study were offered a redo procedure based on the presence of persistent disabling symptoms, as well as increased risk of neurological events, despite adequate antiplatelet therapy and anticoagulation.
Conclusions: A second percutaneous interatrial septal occluder is feasible in those patients with significant residual shunting following initial closure.
Introduction
Patent foramen ovale (PFO) is a common congenital condition and is estimated to occur in up to 25-30% of individuals1,2. Although usually asymptomatic, a number of clinical manifestations have been recognised. These include cryptogenic stroke3, migraine with aura4, decompression sickness5 and platypnea-orthodexia syndrome6. Current studies are underway in an attempt to define clearly which of these patient groups are likely to benefit from formal PFO closure by percutaneous means.
By far the most experience with percutaneous PFO closure has been obtained in patients who have sustained cryptogenic strokes. Since the first description in 19927 a number of randomised control trials have been performed to ascertain whether PFO closure results in a reduction in subsequent neurological events. Success rates in eliminating a right-left-shunt range from 81%8 to greater than 90%9 whilst the rate of recurring strokes and transient ischaemic attacks is estimated to be between 0-4.9% of cases. Major complications from percutaneous closure are uncommon (1.4%) whilst minor complications occur in approximately 7.9% of cases10.
As technological advancements occur with interatrial septal occluder devices, complication rates and persistent shunting rates are likely to decrease. Until this time a specific challenge for operators is to determine the best modality of treating patients with an occluder device in situ and significant persistent right-to-left shunting.
We report four such cases in where a residual shunt was detected after initial PFO closure. The degree of shunting was defined to be small if 3-9 contrast bubbles appeared, moderate if 10-30 contrast bubbles appeared, and large if more than 30 contrast bubbles appeared in the left atrium after a bubble contrast transthoracic echocardiogram (BCTTE)11. In all cases redo PFO closure was technically feasible in reducing the magnitude of the shunt.
Case 1
A 32-year-old lady presented with exertional breathlessness and symptoms of haemiplegic migraine. She was otherwise a fit and well lady with no other significant medical comorbidities. As part of her investigations she underwent a bubble contrast transthoracic echocardiogram (BCTTE). This demonstrated the presence of a very large right-to-left shunt at the level of the interatrial septum – there was complete opacification of the left ventricle without Valsalva provocation. The lady was diagnosed as having a very large patent foramen ovale with free right-to-left flow and in view of her exertional breathlessness after discussion was scheduled for a PFO closure. At the time of her procedure intracardiac echo confirmed a large PFO with 18 mm septum primum excursion on balloon palpation of the defect. This was closed using a 30 mm HELEX device (Gore Medical, Flagstaff, AZ, USA) without complication. The lady was advised to remain on a course of aspirin and clopidogrel for a total duration of six months.
At six months the lady continued to report symptoms of marked exertional dyspnoea and ongoing migraine upon cessation of antiplatelet therapy. She was therefore advised to continue these for a total period of one year to provide an adequate time period for the initial defect to seal. At this time a repeat BCTTE was performed. This demonstrated a well-seated HELEX closure device but with a large residual right-left shunt both at rest and with Valsalva provocation (Figure 1a). Transoesophageal echocardiography confirmed a defect to be located at the superior aspect of the device from the left atrial side.
Given the lady’s ongoing disabling symptoms, she was listed for a redo PFO closure. Under TEE guidance, the pre-existing Helex device was crossed at its superior pole and a 25 mm Amplatzer Cribriform device (AGA Medical, Plymouth, MN, USA) was deployed either side of the HELEX device. This effectively compressed the device onto the interatrial septum (IAS) (Figure 1b). Periprocedural contrast assessment of the IAS demonstrated no persistent shunt.
At follow-up the patient’s symptoms of breathlessness and migraine had settled. Clopidogrel was initially discontinued but was later recommenced following a recurrence of migraine headaches. This led to a complete resolution of the patient’s symptoms. Repeat BCTTE demonstrated no residual shunt at rest and a Grade II residual shunt upon Valvsalva provocation at the level of the device closure (Figure 1c). Since the patient was asymptomatic no further intervention was planned pending a further BCTTE scheduled for in nine months time.
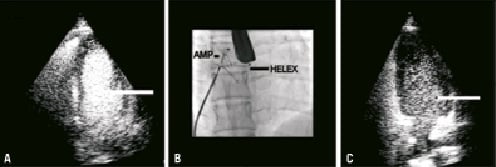
Figure 1. Case 1. A) BCTTE demonstrating the residual shunt across the interatrial septum following initial device closure. B) The fluoroscopic appearance following the redo PFO closure. C) BCTTE demonstrating a residual shunt at the level of the redo PFO closure.
Case 2
A 48-year-old lady working as a professional diver experienced an episode of decompression sickness upon surfacing from a dive. As part of her investigations she underwent a BCTTE. This demonstrated the presence a large PFO with complete opacification of the left ventricle upon Valsalva provocation. Given that her livelihood depended upon her ability to dive, she was listed for a PFO closure. At the time of her procedure intracardiac echocardiography demonstrated standard simple PFO anatomy with a 7 mm tunnel. This was closed with a 23 mm BioStar (NMT Medical, Boston, MA, USA) device without complication. The lady was advised to remain on a course of aspirin and clopidogrel for a total duration of six months.
At six weeks a repeat BBCTE was performed in order to facilitate an early return to work. This demonstrated a residual moderate right-to-left shunt upon Valsalva provocation. This was again reconfirmed at six months (Figure 2a). The patient was accordingly listed for a redo PFO closure.
At the time of her procedure, periprocedural TEE demonstrated a tiny serpiginous defect associated with some passage of contrast into the left atrium. This proved difficult to cross, requiring a 0.014” wire and a transseptal sheath to gain access to the left atrium. A 0.035” guidewire was then introduced to the left upper pulmonary vein and was exchanged for the delivery sheath thereafter. A 25 mm Amplatzer PFO device was then deployed comfortably on either side of the defect sandwiching the previous BioStar device (Figure 2b).
At follow-up, a further BBCTE confirmed that the redo PFO closure had been successful with no shunt at the level of the device closure (Figure 2c).
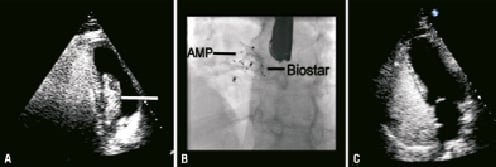
Figure 2. Case 2. A) BCTTE demonstrating the residual shunt across the interatrial septum following initial device closure. B) The fluoroscopic appearance following the redo PFO closure. C) BCTTE demonstrating an intact IAS following redo PFO closure.
Case 3
A 59-year-old lady with a long-standing history of migraine with aura presented with a left sided sensory disturbance associated with white matter change on a cerebral magnetic resonance imaging scan. A BCCTE demonstrated the presence of a large PFO and the patient was referred for a neurological opinion. In the absence of additional risk factors for a cerebrovascular accident it was felt that the PFO was a likely source for her symptoms and she was accordingly referred for device closure.
At the time of her procedure, intracardiac echocardiography confirmed the presence of a moderate sized PFO. This was closed with a 20 mm HELEX device without complication. The patient was advised to remain on aspirin and clopidogrel therapy for a period of six months.
At six months a repeat BCTTE demonstrated a moderate residual shunt across the IAS upon Valsalva provocation. This finding was reconfirmed at one year (Figure 3a). The lady continued to report ongoing symptoms of migraine with aura and was accordingly listed for a redo PFO closure.
At the time of her procedure, transoesophageal echocardiography demonstrated that there was a shunt present through the longitudinal axis of the device between the helices. Given this, a transseptal puncture was performed at the inferior aspect of the device and a 30 mm Amplatzer PFO cribriform device was deployed across the defect. This effectively apposed the HELEX device leaving no residual defect across the IAS (Figure 3b).
At follow-up BCCTE a trivial residual shunt was detected at the level of the device closure (Figure 3c). Since the patient had noticed a significant improvement in her symptoms, no further intervention was planned pending a further BCTTE in six months time. This subsequently demonstrated no residual shunting.
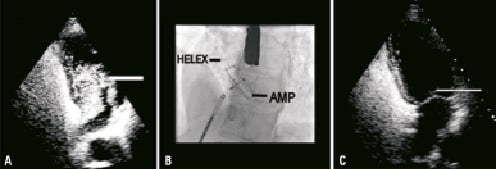
Figure 3. Case 3. A) BCTTE demonstrating the residual shunt across the interatrial septum following initial device closure. B) The fluoroscopic appearance following the redo PFO closure. C) BCTTE demonstrating a minor residual shunt at the level of the redo PFO closure.
Case 4
A 42-year-old gentleman presented with neurological symptoms consistent with ischaemic cerebrovascular accidents. He was normally a fit and well individual with no other significant medical comorbidities. A CT scan demonstrated the presence of an area of cerebral infarction in the left middle cerebral artery territory whilst an MRI scan also demonstrated an additional area within the cerebellum. In the absence of other risk factors the patient underwent a BCTTE. This demonstrated the presence of an aneurysmal interatrial septum with a large right-to-left shunt both at rest and also upon Valsalva provocation. The patient was accordingly referred for device closure as part of the PC-Trial (Patent foramen ovale and Cryptogenic embolism Trial).
At the time of the procedure the large PFO was confirmed and was closed under echocardiographic guidance with a 25 mm Amplatzer PFO occluder with no complications. The patient was advised to remain on a course of aspirin lifelong and clopidogrel for three months.
At part of the trial protocol the patient underwent a routine TEE to assess the device patency at one year. This demonstrated a large right-to-left shunt without Valsalva provocation located over the caudal rim (Figure 4a). Given the magnitude of the residual shunt the patient was listed for a redo PFO closure.
At the time of his procedure transoesophageal echocardiography, intracardiac echocardiography and direct interrogation with a multipurpose catheter demonstrated the passage of contrast running up from the base of the device cranially into the left atrium. Given the orientation of the defect, a transseptal puncture was performed at the base of the device following which a 25 mm Amplatzer PFO device was deployed. This effectively sealed the defect at the inferior margin (Figure 4b).
At follow-up a BCTEE confirmed that the redo PFO closure had been successful with no shunt evident at the level of the device closure (Figure 4c).
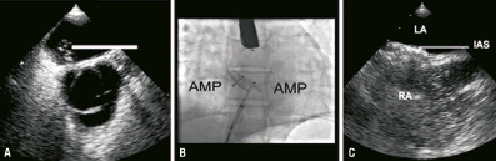
Figure 4. Case 4. A) BCTTE demonstrating the residual shunt across the interatrial septum following initial device closure. B) The fluoroscopic appearance following the redo PFO closure. C) BCTTE demonstrating an intact IAS following redo PFO closure.
Discussion
During the study period 101 patients underwent PFO closure at our institute. Eighty-one (81) patients (80%) underwent PFO closure for cerebrovascular events, 12 patients (12%) for migraine with aura, eight patients for systemic embolisation (8%), three patients (3%) for decompression illness and one patient underwent PFO closure for platypnea-orthodexia syndrome. Of these, four patients (4%) had at least a moderate residual shunt at follow-up. Irrespective of the initial device, redo closure was technically feasible in all cases although slight variations in methodology were required. In the two patients who had an initial closure with the HELEX device, there was a smaller persistent shunt following the second closure attempt. In the patients who had an initial closure with an Amplatzer and BioStar device, there was no residual shunting following the second closure attempt. This variation likely represents differences in the localisation and magnitude of the shunt along with heterogeneous characteristics of the initial closure device.
All patients had at least a moderate residual shunt evident on echocardiographic evaluation at > 6-month follow-up. Although it remains unclear as to what the best therapeutic approach to treat these patients is, anecdotal reports of redo PFO closure have been described in the literature12. The patients in the current study were offered a redo procedure based on the presence of persistent disabling symptoms and also on the increased risk of neurological events (four fold) even despite adequate antiplatelet therapy and anticoagulation13. We did not offer a redo PFO closure procedure to patients with only a minor residual shunt where the shunt was likely to regress further over time14 and where the risk of thromboembolic events is likely to be small15.
Previous studies have identified that patients with an interatrial septal aneurysms undergoing PFO closure with an Amplatzer PFO closure are more likely to have residual shunting at six month follow-up. Greutmann et al8 identified the presence of residual shunting in 19% of patients undergoing PFO closure and identified that that there was an increased frequency in patients with an interatrial septal aneurysm (27%) when compared with those patients without (8%). The current study was not designed to assess interatrial septal and PFO morphology to indicate which patients were likely to have a residual shunt at six months. It is likely however that with larger series, factors such as the presence of a large interatrial septal aneurysm and a long tunnel length may prove to be important considerations. In the current series residual shunting was noted in a variety of different devices and the study was not designed to evaluate individual device specifications. It remains yet to be determined whether this was an important predictor of residual shunting.
There are few similar reports in the literature of successful late redo PFO closure. Scherwzmann et al12 assessed the patency of percutaneous occlusion at six months in 268 patients. They noted complete PFO occlusion in 212 patients (79%), a minor residual shunt in 30 patients (11%) and a moderate-large residual shunt in 28 (10%) patients. In 10 patients with significant residual shunting redo PFO closure was successful in nine cases (90%). Our findings are consistent with that of Scherwzmann et al12 and indicate that redo PFO closure with a secondary device is technically feasible but also that it is associated with a high success rate in reducing patient symptoms.
In all of our cases we elected to use an Amplatzer PFO device as the secondary closure device. This was a standard Amplatzer PFO occluder in two cases and Amplatzer PFO Cribriform occluder in the remaining two. This was to enable repositioning and retrieval of the device as required. The Amplatzer Cribriform device also had the additional benefit of having a small intra-waist connecting diameter with equal sized left- and right-sided discs to facilitate the covering of more than one defect.
Conclusion
A second percutaneous interatrial septal occluder in feasible in those patients with significant residual shunting following initial closure.

