A 49-year-old woman, with a history of cryptogenic stroke and percutaneous patent foramen ovale (PFO) closure using a bioabsorbable device with a diameter of 28 mm, was seen in our outpatient clinic for contrast transthoracic echocardiography (TTE) at three-year follow-up. Earlier contrast TTE had shown a small right-to-left shunt. Currently, her clinical investigation was unremarkable. The TTE showed a good device position, without thrombi on the device. Colour Doppler showed no signs of shunting, but using contrast (Figure 1A) a severe right-to-left shunt was diagnosed during Valsalva. Under local anaesthesia and intracardiac echocardiographic guidance, the residual shunt was closed percutaneously using a 27-30 mm Occlutech® Figulla® device (Occlutech International AB, Helsingborg, Sweden) (Figure 1B and Figure 1C). There were no complications and contrast TTE before discharge showed only small residual shunting with a good device position (Figure 1D). Residual shunting is common in patients who had received a bioabsorbable device for PFO closure1. Due to the increased risk of recurrent stroke in the presence of a large residual shunt, treatment might be necessary and can be provided by oral anticoagulation therapy, surgical or percutaneous closure2. Little is known about the safety and efficacy of these therapies in this specific situation. Successful implantation of a second device has been described in a small number of patients showing a good periprocedural result3,4.
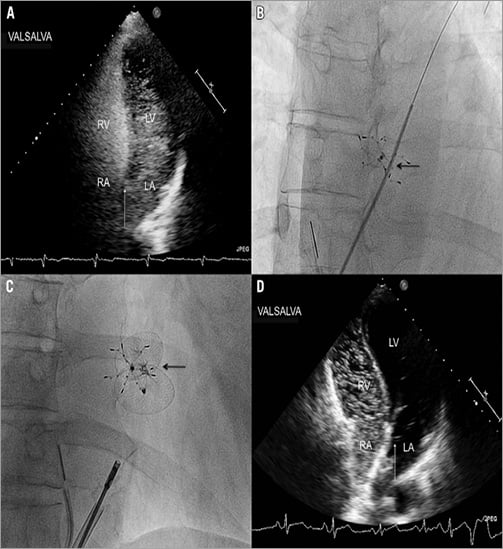
Figure 1. A) Contrast transthoracic echocardiogram (TTE) at three-year follow-up. During Valsalva a severe right-to-left shunt is visible (arrow). B) Fluoroscopic view of the bioabsorbable device (arrow) with the catheter passed through the residual opening. C) Fluoroscopic view of the bioabsorbable device and the Occlutech Figulla device (arrow). D) Contrast TTE 1 day after intervention. During Valsalva a small right-to-left shunt is visible (arrow).
We report a case of a successful second percutaneous device implantation, because of a severe residual shunt, after a previous percutaneous PFO closure with a bioabsorbable device.
Conflict of interest statement
The authors have no conflicts of interest to declare.

