Abstract
Aims: To evaluate the relationship between the anatomic features of the fossa ovalis (FO) and residual right-to-left shunt (RLS) after percutaneous patent foramen ovale (PFO) closure with AMPLATZER PFO occluder devices.
Methods and results: FO anatomic features were assessed by intracardiac echocardiography in 127 patients with large RLS at contrast-enhanced transcranial colour Doppler (TCCD) undergoing percutaneous PFO closure with an AMPLATZER device. Residual RLS was evaluated by TCCD three and 12 months after the procedure. PFO closure was successful in all but two patients. At TCCD, a significant residual RLS (grade ≥2) was observed in 27 (21.6%) and 17 (13.6%) patients at three and 12 months, respectively. Larger baseline RLS, presence of atrial septal aneurysm, greater longitudinal and transverse FO dimensions, and use of larger devices were associated with significant residual RLS. At multivariate analysis, the presence of atrial septal aneurysm (OR 7.6; 95% CI: 1.38-42.35; p=0.02) and longitudinal FO dimension >20.8 mm (OR 8.5; 95% CI: 1.55-46.95; p=0.014) were identified as independent predictors of significant residual RLS at 12 months.
Conclusions: Our study suggests that a large FO and the presence of atrial septal aneurysm are independent predictors of persistent residual RLS after PFO closure with AMPLATZER devices.
Abbreviations
ASA: atrial septal aneurysm
FO: fossa ovalis
ICE: intracardiac echocardiography
MRI: magnetic resonance imaging
PFO: patent foramen ovale
RLS: right-to-left shunt
TCCD: transcranial colour Doppler
TIA: transient ischaemic attack
Introduction
Patent foramen ovale (PFO), especially when associated with atrial septal aneurysm (ASA), has been identified as a potential risk factor for cryptogenic stroke, and percutaneous closure of PFO has been proposed as an effective therapeutic option for secondary prevention of paradoxical embolism1. Procedural success rates, in terms of absence of significant residual shunting, vary widely across studies, because of differences in device type, interventional technique, ultrasonographic method for shunt assessment, and fossa ovalis (FO) anatomy2-8. Our objective was to evaluate the relationship between residual right-to-left shunt (RLS), assessed by transcranial colour Doppler (TCCD), and the morphological characteristics of FO, visualised with rotational intracardiac echocardiography (ICE), in patients undergoing percutaneous PFO closure with an Amplatzer occluder device, presenting with significant RLS and cryptogenic cerebral ischaemia, peripheral embolism, or migraine with subclinical brain lesions.
Methods
PATIENT POPULATION
From June 2003 to May 2010, 127 consecutive patients <60 years old underwent ICE-guided percutaneous PFO closure with an AMPLATZER™ PFO occluder (St. Jude Medical, Plymouth, MN, USA) at our institution. Patients were selected according to the following criteria: (i) presence of PFO with a large spontaneous or inducible RLS during TCCD; (ii) history of cryptogenic cerebral ischaemia (transient ischaemic attack [TIA] or stroke), migraine associated with subclinical brain lesions, or peripheral embolism; (iii) exclusion of other cardiac, aortic or cerebrovascular causes of cerebral ischaemia. All patients underwent laboratory screening for acquired and congenital thrombophilia, including antithrombin III, antiphospholipid antibodies (anticardiolipin, lupus anticoagulant), protein C, free protein S, homocysteine, and Factor V Leiden and prothrombin mutations. The study was approved by our institutional review board committee and all patients gave written informed consent.
CONTRAST-ENHANCED TRANSCRANIAL COLOUR DOPPLER
TCCD was performed according to the recommendations of the Venice Consensus Conference9, using an ATL HDI 5000 system (ATL Ultrasound Inc., Bothell, WA, USA), with an injection of 10 ml of agitated saline. RLS was quantitatively graded using a six-level scale, as previously described10: 0=absence of shunt (no microembolic signals); 1=latent shunt of mild degree (1 to 20 microembolic signals) after Valsalva manoeuvre; 2=latent shunt of moderate degree (>20 microembolic signals and no curtain, defined as a shower of microembolic signals where a single signal cannot be identified, after Valsalva manoeuvre); 3=latent shunt of high degree (curtain after Valsalva manoeuvre); 4=permanent shunt of mild/moderate degree (>10 microembolic signals at rest and curtain after Valsalva manoeuvre); 5=permanent shunt of high degree (curtain at rest). Only patients with a RLS of grade ≥3 were included in the study.
ICE-GUIDED PERCUTANEOUS PFO CLOSURE
Percutaneous PFO closure was performed under rotational ICE guidance and local anaesthesia in the right (for device access) and left (for ICE catheter access) groin, without sedation. ICE was performed using a commercially available mechanical 9 Fr, 9 MHz Ultra ICE catheter-based ultrasound transducer (EP Technologies, Boston Scientific Corp., San José, CA, USA), connected to an imaging console. Depth of penetration, axial and lateral resolution were 5 cm, 0.27 mm, and 0.26 mm, respectively. The Ultra ICE catheter was introduced percutaneously into the left femoral vein through a 55° precurved polyethylene venous sheath (Convoy; EP Technologies, Boston Scientific Corp.). Two standardised orthogonal sections were used to obtain ICE-derived measurements of FO for appropriate device selection: a) the transverse section on the aortic valve plane (ICE-aorta) without catheter rotation and with the transducer positioned at 0° between the 6th and 7th intervertebral thoracic discs; b) the longitudinal section on the 4-chamber plane (ICE-4-chamber), obtained with the 55° precurved introducer sheath advanced up to the end of the catheter and turned backward and leftward for longitudinal scanning of the atrial septum. The latter view was also used to monitor device deployment. The AMPLATZER device size was chosen large enough to ensure complete FO coverage11; no balloon sizing of the defect was performed. ICE images were stored on a hard disk at a rate of 50 frames/sec, and all measurements were obtained off-line at atrial diastole. The diagnosis of ASA was made when the atrial septum exhibited an excursion of ≥10 mm with a base diameter of ≥15 mm. Tunnel length was defined as the maximum overlap of the septum primum and septum secundum. The presence of a prominent Eustachian valve or Chiari’s network was also reviewed.
FOLLOW-UP
All patients received dual antiplatelet therapy with aspirin (100 mg/day) and clopidogrel (75 mg/day) for three months, and then aspirin alone for three months. A transthoracic echocardiogram and chest x-ray were obtained 24 hours after the procedure, and the transthoracic echocardiogram was repeated at three and 12 months. Transoesophageal echocardiogram was planned only in the case of a suspected procedure-related complication. Quantitative assessment of residual RLS was performed with TCCD at three and 12 months. A residual grade ≥2 RLS was considered significant. Patients with significant residual RLS underwent contrast transthoracic echocardiography to confirm the presence of intracardiac shunt. All patients were followed up clinically for the occurrence of death, recurrent ischaemic stroke, TIA, or peripheral embolism. Patients with suspected recurrent cerebrovascular events were re-examined by a neurologist, and magnetic resonance imaging (MRI) of the brain was scheduled.
STATISTICAL ANALYSIS
Data are reported as mean±standard deviation. The Kolmogorov-Smirnov test was performed to verify whether all variables followed a normal distribution. Fisher’s exact test was used for categorical variables, and the t-test or Mann-Whitney U test was used for scale variables if data followed normal or non-normal distribution, respectively. For scale variables that showed a significant difference between the two groups (presence or absence of significant residual RLS), receiver-operating characteristic (ROC) cut-off values were obtained according to the best compromise between sensitivity and specificity. Multivariate logistic regression analysis with stepwise backward elimination was accomplished, considering the presence of significant residual RLS as dependent variable. Sex, age, and all variables presenting a significant value at univariate analysis were entered into the model, and odds ratios with 95% confidence intervals were calculated. Statistical significance was set at p<0.05 (two-tailed) for all tests. All data were analysed using SPSS software (version 11.0; SPSS Inc., Chicago, IL, USA).
Results
PATIENT CHARACTERISTICS AND CLINICAL FOLLOW-UP
Percutaneous PFO closure was successful in all but two patients with large ASA, in whom firm anchorage of the device could not be achieved. Baseline characteristics of patients are listed in Table 1. ASA was present in 39 (31.2%) patients, the prevalent indication for PFO closure was prior stroke/TIA (68%), and more than half of the patients received a 25 mm device (58.4%). Three patients developed minor haematomas in the left groin. At postoperative transthoracic echocardiography, there was no evidence of device malposition or thrombosis. At a mean follow-up of 19±9 months, clinical relapse occurred in three patients (2.4%): (i) a 35-year-old woman experiencing a TIA, lasting 15 minutes, three months postoperatively; (ii) a 49-year-old woman with transient visual abnormalities, two months after the closure; (iii) a 44-year-old woman with transient loss of strength in arm, two months postoperatively. It is worth mentioning that the first patient still had significant residual RLS (grade 3) at the first TCCD control, while the second and the third had no residual RLS (grade 0) at three months. In all three patients, urgent diffusion-weighted MRI did not show new lesions compared with previous examinations. The remaining patients were clinically asymptomatic.
RESIDUAL SHUNT
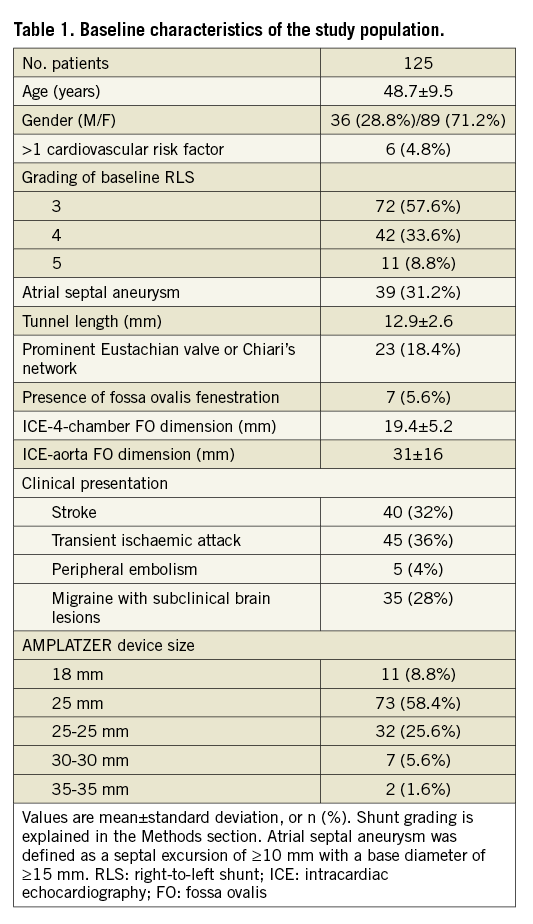
In the overall population, TCCD showed a significant residual RLS (grade ≥2) in 27 (21.6%) and 17 (13.6%) patients at three and 12 months, respectively (Table 2). This patient subgroup more frequently had a larger RLS at baseline (grade 4 or 5 vs. grade 3, p<0.001 for both; Table 2). As regards PFO morphology, patients with significant residual RLS more frequently had ASA (subgroup with significant shunt vs. non-significant shunt: 17/27 [63%] vs. 22/98 [22.4%] and 14/17 [82.3%] vs. 25/108 [23.1%] at three and 12 months, respectively, p<0.001 for both). TCCD results at three and 12 months with respect to the presence or absence of ASA are depicted in Figure 1. The subgroup with significant residual RLS exhibited greater ICE-derived longitudinal and transverse FO dimensions and more frequently received a >25 mm device (Table 2).
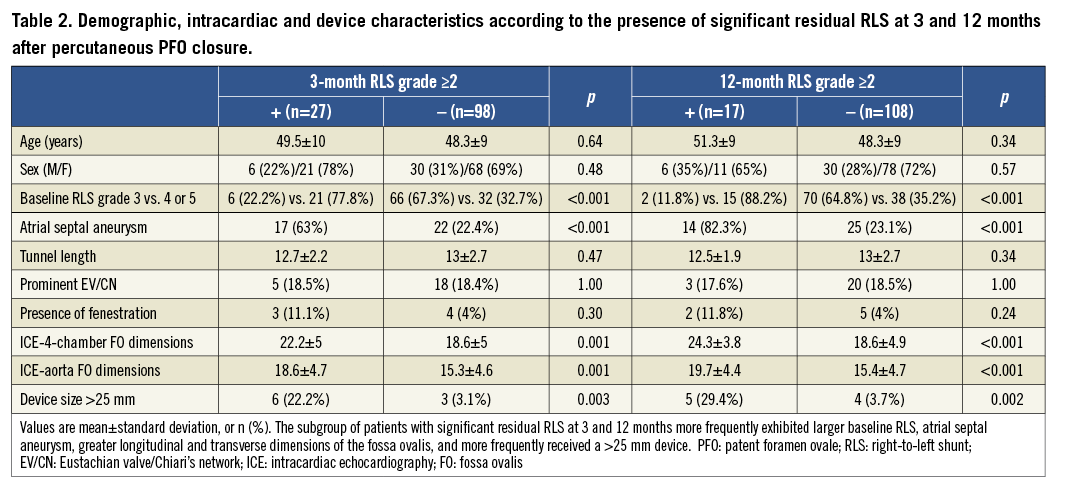
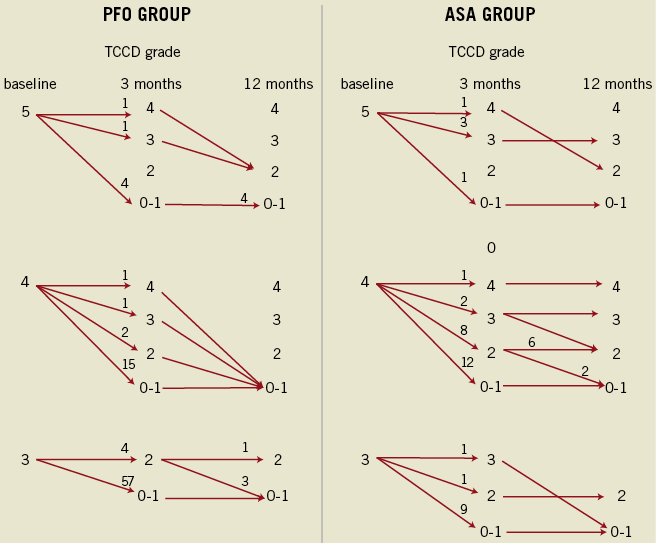
Figure 1. Transcranial colour Doppler findings at 3 and 12 months with respect to the presence or absence of ASA. Numbers near the arrowheads indicate number of patients. Closure rates improved over time. Unlike the ASA group, few patients in the PFO group had significant residual shunt (grade ≥2) at 12 months. PFO: patent foramen ovale; ASA: atrial septal aneurysm; TCCD: transcranial colour Doppler
The ICE-4-chamber and ICE-aorta measurements were highly and directly correlated (r=0.9, p<0.001), and both increased in accordance with grading of baseline RLS (RLS grade 3: ICE-4-chamber 17.9±5.0, ICE-aorta 14.7±4.7; RLS grade 4: ICE-4-chamber 21.1±4.5, ICE-aorta 17.3±4.2; RLS grade 5: ICE-4-chamber 22.7±5.7, ICE-aorta 19.5±5.5; p for trend<0.001 for both; Figure 2). Patients with ASA showed significantly greater longitudinal (24.4±3.7 vs. 17.1±4.1) and transverse (20.2±4.3 vs. 14.0±3.7) FO dimensions than patients without ASA (p<0.001 for both). In addition, the presence of ASA was significantly correlated with a larger baseline RLS (grade 4 or 5 vs. grade 3; p<0.001). Patients who received a >25 mm device (all with ASA) showed greater longitudinal (26.8±4.1 vs. 18.8±4.8) and transverse (21.8±5.2 vs. 15.5±4.5) FO dimensions than patients who received a <25 mm device (p<0.001 for both).
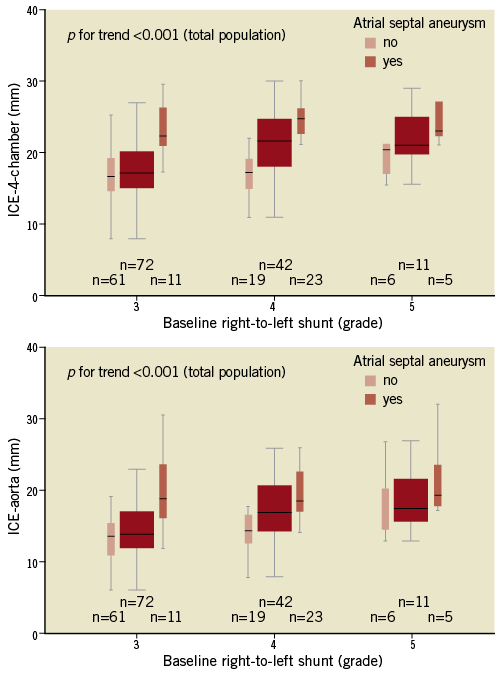
Figure 2. Fossa ovalis dimensions with respect to baseline right-to-left shunt and presence of ASA. ICE-derived longitudinal and transverse dimensions of the fossa ovalis increased in accordance with grading of baseline right-to-left shunt (p for trend <0.001 for both). Patients with ASA showed significantly greater longitudinal and transverse dimensions of the fossa ovalis than patients without ASA (p<0.001 for both). ICE: intracardiac echocardiography; ASA: atrial septal aneurysm
Interestingly, a very large residual RLS (grade ≥3) was observed in 12 patients (9.6%; 8 with ASA) at three months, and only in three patients (2.4%; all with ASA) at 12 months.
Using ROC curve analysis, optimal cut-off values of ICE-derived measurements of FO were obtained to predict significant residual RLS. An ICE-4-chamber dimension of >20.8 mm was found to have a sensitivity and specificity of 95% and 71% (area under the curve=0.83), respectively, and an ICE-aorta dimension of >16.9 mm was found to have a sensitivity and specificity of 77% and 71% (area under the curve=0.78), respectively.
At multivariate analysis, only the presence of ASA (OR 7.6; 95% CI: 1.38-42.35; p=0.02) and an ICE-4-chamber dimension of >20.8 mm (OR 8.5; 95% CI: 1.55-46.95; p=0.014) were independently and directly correlated with the presence of residual RLS at 12 months.
Discussion
The presence of PFO has consistently been shown to be associated with cryptogenic stroke. In addition, the anatomic features of FO (e.g., presence of ASA, prominent Eustachian valve, or large RLS) seem to play a major role in the recurrence of ischaemic events1. Percutaneous PFO closure has been suggested as a safe and effective therapeutic option for secondary prevention of cerebral events likely due to paradoxical embolism. Observational studies have shown that percutaneous PFO closure is effective in reducing clinical and radiological recurrence of cerebral events compared with medical therapy1,12-14. The technical success is primarily related to the absence of significant residual RLS, which has been identified as a potential risk factor for recurrent cerebral events1. To date, the recently published CLOSURE I15 is the only randomised study that has compared percutaneous PFO closure with antithrombotic therapy (anticoagulant or antiplatelet agents). The authors did not find any significant differences in the primary combined endpoint of stroke, TIA and neurological death (5.5% in the closure group vs. 6.8% in the medical therapy group, HR 0.78; 95% CI: 0.45-1.35; p=0.44). Despite its many strengths, CLOSURE I also had some limitations: (i) admission criteria were not restrictive as regards shunt quantification (approximately 50% of recruited patients had trace shunt, defined as the appearance of 1-10 bubbles at transoesophageal echocardiography after Valsalva manoeuvre); (ii) the sample size was relatively small compared with the very low rate of events; (iii) the definition of successful PFO closure (patients with none or trace residual shunt) was rather broad, considering the admission criteria. Although CLOSURE I failed to confirm a favourable effect of percutaneous PFO closure in preventing recurrent neurological events, the possibility of beneficial effects cannot yet be excluded16, especially in selected patients (e.g., PFO with large RLS or ASA).
Our study aimed to evaluate the procedural success, in terms of residual RLS, of ICE-guided percutaneous PFO closure with AMPLATZER devices in a cohort of 125 patients with high-grade RLS at baseline.
In previous studies, the rate of residual RLS at 12 months after percutaneous PFO closure varied considerably, ranging from 4.5%5 up to 34%17. Comparison of results across studies is not possible because of differences in inclusion criteria, device type, echocardiographic techniques (contrast-enhanced transthoracic echocardiography, transoesophageal echocardiography, or TCCD), follow-up duration (rarely exceeding six months), and the absence of systematic quantitative pre- and post-procedural evaluation of RLS.
The present study has at least four methodological strengths. First, entry criteria were very restrictive, and only patients with large RLS (high-degree shunt after Valsalva manoeuvre) were selected for PFO closure. In addition, technical success was defined as mild or absent shunting after Valsalva manoeuvre. Second, only AMPLATZER devices were used, selecting the appropriate size to ensure complete FO coverage. Third, a six-level scale was used for quantitative assessment of RLS by TCCD. Although contrast-enhanced transoesophageal echocardiography allows a diagnosis of intrapulmonary RLS, a direct visualisation of FO, a demonstration of the exact mechanism of residual RLS, and an accurate evaluation of complications (such as device thrombosis or erosion of surrounding anatomic structures), it is a semi-invasive imaging technique and provides a semi-quantitative measure of RLS; moreover, Valsalva manoeuvre may be difficult to perform during transoesophageal echocardiography. Similarly, transthoracic contrast echocardiography provides a semi-quantitative shunt assessment and images may be suboptimal, leading to potential underestimation of shunt size. TCCD has the advantage of being non-invasive and allows quantitative shunt assessment also during Valsalva manoeuvre. Fourth, the systematic use of mechanical rotational ICE for the study of FO anatomy enables the display of truly orthogonal and large field two-dimensional echocardiographic planes, with images very similar to MRI and anatomical sections18.
In our selected and homogeneous cohort of patients, closure rates were 78.4% at three months and 86.4% at 12 months. Similarly to other studies5, closure rates also improved over time, likely due to device endothelialisation. At univariate analysis, baseline RLS grade 4 or 5 vs. 3, the presence of ASA, ICE-derived longitudinal and transverse FO dimensions, and use of large devices (>25 mm) were found to be associated with significant residual RLS. However, at multivariate analysis, only the presence of ASA and the ICE-derived longitudinal dimensions of FO >20.8 mm were identified as independent predictors of significant residual RLS.
As regards ASA, our data are consistent with previous observations5,7,19. Of note, only a few studies have systematically evaluated PFO morphology using transoesophageal echocardiography20 and ICE21. Optimal management of patients with concomitant PFO and ASA remains a difficult challenge, because of complex septal anatomy (large FO, mobile structures, high-degree RLS, single or multiple fenestrations) that frequently affects the orientation of the PFO, potentially lowering procedural success even in the presence of a relatively stable and firm device. The optimal procedural strategy in patients with PFO and ASA remains a matter of debate among interventionalists, with some choosing to cover only the PFO with a small device and others opting for larger devices. We preferred to implant a large occluder device to cover the entire aneurysm22.
We found an independent correlation between ICE-4-chamber FO dimensions (the major axis of FO) and significant residual RLS at 12 months. This novel finding may be explained by the difficulties in covering the largest defects. Previous reports have evidenced a significant direct correlation between device size and residual RLS4. In our study, this observation was confirmed on univariate but not on multivariate analysis, after correction for potentially confounding factors. Device size selection is usually driven by anatomical features, such as morphological characteristics and dimensions of surrounding structures (aorta, Chiari’s network, or Eustachian valve), and by the FO itself. The great majority of previous studies did not take into account echocardiographic data, and the use of a large device may partially be a surrogate marker for large FO. In other words, according to our results, residual RLS is not associated with a larger device per se, but rather with FO dimensions that influence the choice of device size.
Our study offers important clinical implications. First, the use of ICE-guided percutaneous PFO closure with an AMPLATZER device is safe and effective in terms of occlusion rates and recurrent embolic events at 12 months. Second, given the high closure rates in patients with simple defects (PFO without ASA or with small FO), our study draws attention to the presence of a more complex anatomy (PFO with ASA and large FO), that represents a warning for the interventional cardiologist because of its independent correlation with significant residual RLS. Owing to the lack of consistent results among the studies comparing different strategies, how the presence of ASA and large FO may influence the procedural approach remains uncertain. This subgroup of patients needs close and longer follow-up after percutaneous PFO closure, also taking into consideration the potential risk of neurological events associated with residual RLS.
The clinical management of patients with significant residual RLS is controversial. A transoesophageal echocardiography examination in these patients may be useful to study the mechanisms of residual shunting. Therapeutic options include pharmacological therapy with antiplatelet or oral anticoagulant drugs, percutaneous closure with a second device, or even surgical repair23. In our series, a large residual RLS (grade ≥3) was observed in only three patients (2.4%), all with ASA and FO dimensions >20.8 mm, who showed a slight improvement compared to baseline RLS. It was decided to continue aspirin therapy indefinitely.
Study limitations
Some study limitations should be acknowledged. The relatively small sample size, especially for patients with ASA, although comparable with other studies, and the short-term follow-up, longer than that of similar studies, may have limited our analysis. In the absence of established guidelines for device selection, our interventional strategy was oriented toward complete coverage of the FO. This choice may have affected the generalisability of our results. In patients with significant residual RLS, intrapulmonary shunt has not been entirely ruled out by TCCD coupled with contrast transthoracic echocardiography. Finally, a systematic transoesophageal echocardiogram could have been more informative and useful for estimating the exact mechanism of residual RLS and for identifying intrapulmonary shunts. Notwithstanding this, we enrolled a highly selected consecutive cohort of patients with large baseline RLS undergoing ICE-guided PFO closure, and our findings may have important clinical implications.
Conclusions
In patients undergoing ICE-guided percutaneous PFO closure with an AMPLATZER device, the presence of ASA and large FO is associated with persistent residual 12-month RLS by TCCD. Further studies are warranted to evaluate the clinical implications of our findings with respect to the optimal interventional strategy.
Conflict of interest statement
The authors have no conflicts of interest to declare.

