Introduction
Percutaneous patent foramen ovale (PFO) closure has proven to be superior to medical therapy for secondary prevention in selected patients with cryptogenic stroke1. Furthermore, percutaneous PFO closure prevents decompression sickness and platypnoea-orthodeoxia syndrome, and may play a role in patients with migraine2. However, despite their recognised efficacy, PFO occluders carry a potential risk of early and late complications and may represent an obstacle for future transseptal left-sided catheter-based interventions3. As the vast majority of PFO closure interventions are carried out for preventive purposes in a young population, the concept of “deviceless” PFO closure has become increasingly attractive and has been tested for over a decade, but with poor results3. Recently, a suture-mediated approach to percutaneous PFO closure has been proposed. Early data demonstrated feasibility in different anatomical conditions, with a high device success rate and no device-related complications45. Nevertheless, post-procedural PFO patency and device-related complications at follow-up have only been assessed using a transthoracic echocardiography (TTE) microbubble test, which provides limited sensitivity, leading to uncertainty regarding actual device performance67. Therefore, we conducted the present study to evaluate the efficacy and safety of suture-mediated PFO closure by transoesophageal echocardiography (TEE), which has been shown to have higher sensitivity than TTE for detection of residual shunt and characterisation of device-related complications1.
Methods
Between July 2017 and July 2020, 80 consecutive patients underwent percutaneous suture-mediated PFO closure with the NobleStitch EL device (HeartStitch) at our institution and were included in this prospective observational registry. The study design is provided in Supplementary Figure 1. Inclusion and exclusion criteria are discussed in Supplementary Appendix 1. TEE was performed at baseline and between 3 and 6 months post procedure to evaluate residual right-to-left shunt (RLS), predictors of device failure, and device-related complications. Details of the TEE examination protocol and procedural steps are illustrated in Supplementary Figure 2 and Supplementary Appendix 2. The primary efficacy outcome was effective PFO closure at TEE follow-up (residual RLS grade ≤1 at microbubble test). Secondary efficacy outcomes were procedural success (correct device delivery followed by negative intraprocedural contrast injection and TTE microbubble test) and complete PFO closure (residual RLS grade 0 at TEE follow-up). Secondary safety outcomes were procedure-related adverse events (access-site complications, bleeding, pericardial effusion or cardiac tamponade, arrhythmias), and in-hospital and follow-up device-related adverse events (device detachment, device embolisation, device thrombosis, or atrial septal damage). Other outcomes and the statistical analysis are discussed in Supplementary Appendix 3 and Supplementary Appendix 4.
Results
Baseline clinical and echocardiographic characteristics
The median PFO tunnel length and width were 10 mm (interquartile range [IQR]=6-12 mm) and 4 mm (IQR=3-5 mm), respectively. Atrial septal aneurysm was encountered in 9% of patients, while a prominent Eustachian valve or Chiari network was identified in 11% of patients. A stretched PFO anatomy was reported in 31% of cases. The microbubble test was positive in all patients during the Valsalva manoeuvre and in 54% of patients at baseline during normal respiration; 44% of patients presented with a moderate RLS (grade 2) and 56% with a severe RLS (grade 3) (Supplementary Table 1).
Procedural and in-hospital outcomes
Percutaneous suture-mediated PFO closure was successfully carried out in 94% of patients. In 6 patients intraprocedural contrast injection and a TTE microbubble test demonstrated significant residual RLS that was addressed by an additional stitch delivery in 1 case, and by AMPLATZER PFO Occluder (Abbott) implantation in the remaining 5 cases. Procedural complications occurred in 8 (10%) patients. No device-related complications were observed. The majority of patients received one-month single antiplatelet therapy at discharge (Supplementary Table 2).
Transoesophageal echocardiography follow-up
Of the 75 successfully treated patients, 65 (87%) underwent 3- to 6-month TEE follow-up. The mean TEE follow-up time was 142±96 days (Supplementary Table 3). The primary efficacy outcome of effective PFO closure occurred in 82% of patients. Complete PFO closure occurred in 62% of patients (Supplementary Figure 3). Overall, a significant reduction in RLS at the TEE microbubble test follow-up was observed (p<0.001). Residual RLS was present in 25 (38%) patients: 13 (20%) grade 1, 6 (9%) grade 2 and 6 (9%) grade 3 (Central illustration).

Central illustration. Suture-mediated percutaneous patent foramen ovale closure with the NobleStitch EL device: efficacy and failure predictors at transoesophageal echocardiography follow-up. PFO: patent foramen ovale; RLS: right-to-left shunt ; TEE: transoesophageal echocardiography
Predictors of ineffective PFO closure
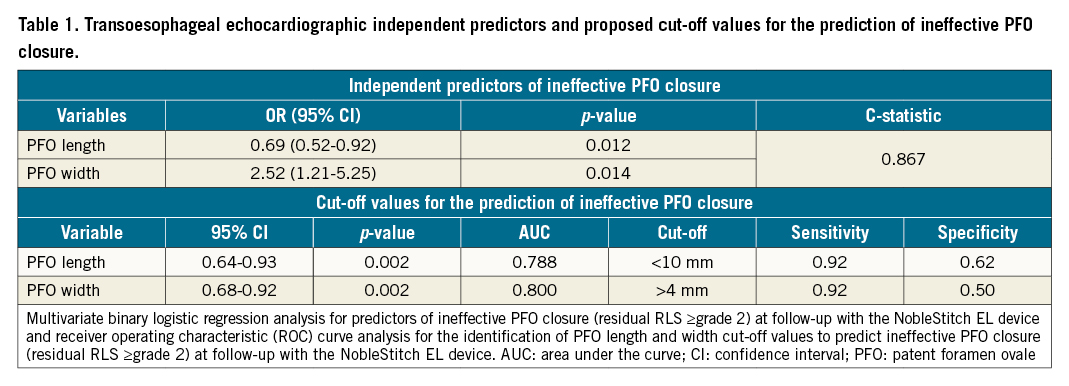
A comparison of baseline clinical characteristics, echocardiographic features and procedural variables was performed between patients with effective PFO closure and those without (Supplementary Table 4). Supplementary Table 5 shows the results of the univariate analysis. Multivariable logistic regression analysis identified PFO length (odds ratio [OR] 0.69, 95% confidence interval [CI]: 0.52-0.92, p=0.012) and PFO width (OR 2.52, 95% CI: 1.21-5.25, p=0.014) as independent predictors of ineffective PFO closure at TEE follow-up, with a good accuracy of the regression model (C-statistic 0.867) (Table 1). Receiver operating characteristic (ROC) curve analysis identified a cut-off of 10 mm for PFO length (area under the curve [AUC] 0.788, sensitivity 92%) and of 4 mm for PFO width (AUC 0.800, sensitivity 92%) for the detection of incomplete PFO closure with the NobleStitch EL device at TEE follow-up (Table 1). No cases of device detachment or embolisation were reported. A case of device thrombosis was documented in a patient with thrombotic diathesis which was managed with oral anticoagulation. A case of atrial septal defect was observed and treated with repeat percutaneous PFO closure using an AMPLATZER PFO Occluder8.
Clinical follow-up
Clinical follow-up was available for all 65 patients who underwent TEE follow-up (Supplementary Table 6).
Discussion
Despite the lack of specific patient selection criteria, suture of the atrial septum with the NobleStitch EL device has shown encouraging results in terms of successful implantation, effective PFO closure and safety45. In this study, we adopted a systematic TEE-based strategy to evaluate device efficacy at 3- to 6-month follow-up in order to improve detection of residual RLS and to identify reliable functional and anatomical predictors of device failure and device-related complications, which should be carefully assessed during the initial clinical experience with every new technology. Effective closure was defined as residual RLS grade ≤1 at TEE follow-up, according to the definition adopted in the majority of clinical trials on PFO closure with traditional devices9. The rate of effective PFO closure with the NobleStitch EL device evaluated at 3- to 6-month TEE follow-up was 82%. This result appears to be in line with previously reported TTE data for the NobleStitch EL, but lower than TEE data for traditional PFO closure devices (Supplementary Table 7). Despite different endpoint definitions derived from different RLS grading scores, and the lack of direct comparisons, the lower rates of effective PFO closure observed with the suture-mediated technique in currently available registries might have been influenced by inadequate patient selection criteria12345. Among baseline TEE anatomical features, multivariable logistic regression analysis identified PFO length and PFO width as independent predictors of ineffective PFO closure at TEE follow-up. PFO length <10 mm and PFO width >4 mm showed 92% sensitivity for the identification of suboptimal anatomy for PFO closure with the NobleStitch EL device (Figure 1). These results highlight the importance of careful anatomical assessment of the fossa ovalis by preprocedural baseline TEE to identify those patients who could benefit the most from suture-mediated PFO closure. Although commonly described as a tunnel, PFO has a semilunar shape that requires multiple 2D views or ideally 3D multiplanar imaging reconstruction to be fully characterised (Supplementary Figure 4). Whether advanced procedural imaging guidance could improve outcomes remains to be determined. Real-time 3D TEE or intracardiac imaging of the fossa ovalis could guide septal suture placement and orientation during the procedure in order to achieve optimal sealing. As immediate post-procedure residual RLS has been shown to predict late shunt after PFO closure, the implementation of intraprocedural TEE assessment at the time of index procedure could help to identify a significant proportion of patients who would have presented with ineffective PFO closure at follow-up10. Finally, our data confirm the safety profile of the NobleStitch EL device, despite an increased sensitivity in device-related complication detection provided by the use of TEE at follow-up, with a rate of device thrombosis and atrial septal damage of 2% at 3 to 6 months.
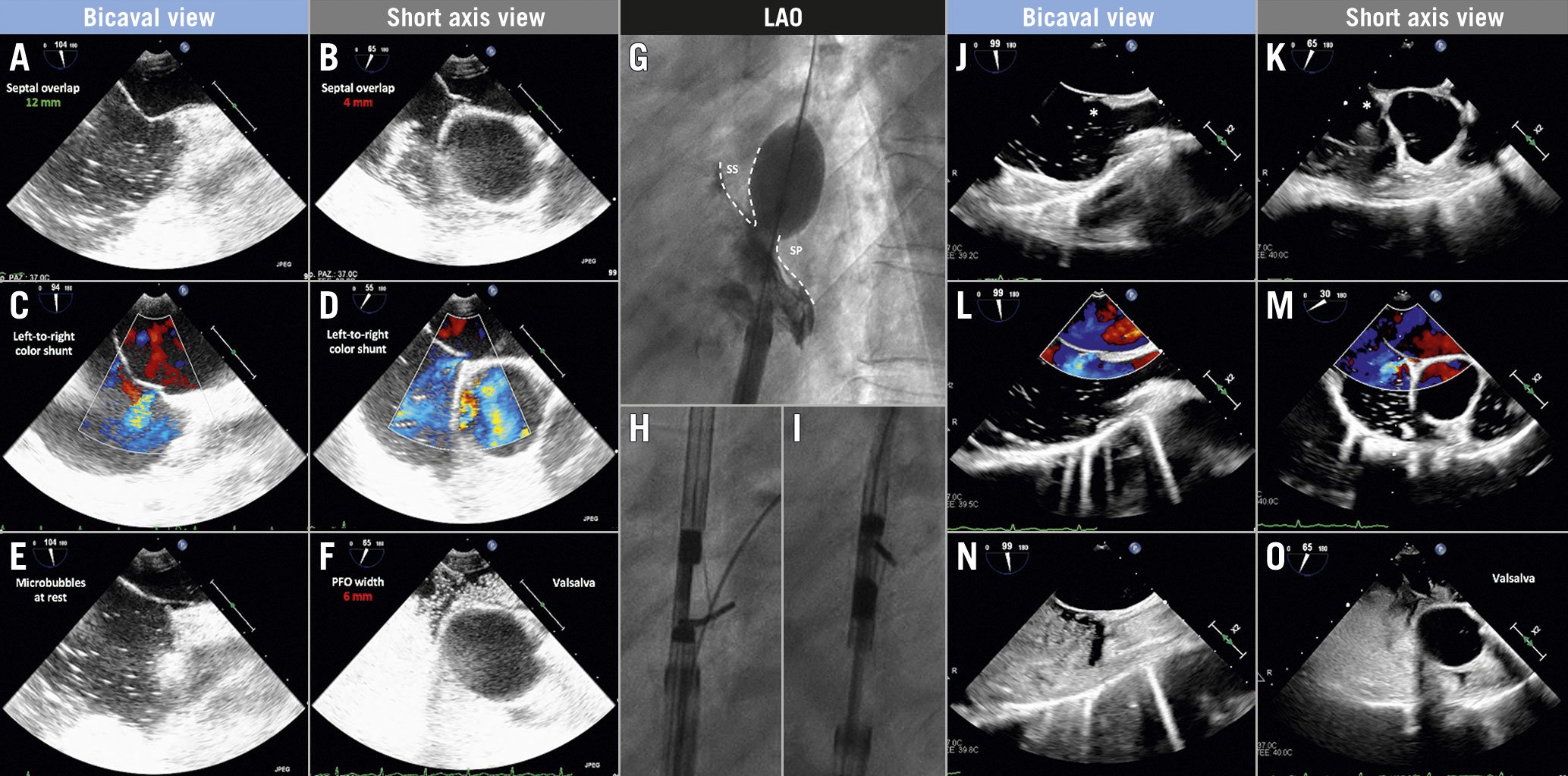
Figure 1. Transoesophageal echocardiographic anatomical assessment of patent foramen ovale (PFO). Baseline TEE anatomical assessment in multiple 2D views showing favourable septal overlap in the bicaval view (A) that reduces to 4 mm in the short-axis view (B), baseline colour left-to-right shunt (stretched PFO) in both views (C & D), microbubble passage at rest even before the complete filling of the right atrium (E) and wide PFO opening with severe right-to-left shunt during Valsalva manoeuvre (F). Sizing balloon interrogation of the atrial septum demonstrating minimum overlap between septum secundum (SS) and septum primum (SP) (G). Percutaneous PFO closure using the NobleStitch device: delivery of septum secundum (H) and septum primum (I) sutures. Follow-up TEE evaluation at three months showing the KwiKnot (asterisk) in place (J & K) and demonstrating incomplete closure of the PFO with residual left-to-right colour shunt (L & M) and residual right-to-left shunt during Valsalva manoeuvre (N & O). LAO: left anterior oblique
Limitations
All the procedures were performed at a single centre by highly experienced operators, thus our results need further confirmation in order to be generalisable. Microbubble injections at baseline and follow-up were performed from the upper extremity, potentially affecting the sensitivity of the test. The lack of another PFO closure device for direct comparison, together with the relatively small sample size and short follow-up length, does not allow the discrimination of efficacy in terms of recurrent cerebrovascular event reduction.
Conclusion
Suture-mediated PFO closure with the NobleStitch EL device currently provides lower rates of effective PFO closure than traditional devices with a favourable safety profile at 3- to 6-month TEE follow-up. PFO width and length at baseline TEE are relevant selection criteria that might improve the outcomes of the procedure. Further studies on larger populations are warranted to assess whether the proposed anatomical selection criteria and procedural imaging could improve the outcomes of percutaneous suture-mediated PFO closure and to establish more fully the role of the NobleStitch EL in the complex landscape of percutaneous PFO closure devices.
Impact on daily practice
Suture-mediated percutaneous PFO closure provides 82% effective PFO closure with a low rate of device-related complications at 3- to 6-month TEE follow-up. PFO length <10 mm and PFO width >4 mm identify patients with an unsuitable anatomy for the procedure.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

