
Abstract
Aims: The aim of this study was to assess the efficacy of a novel percutaneous “deviceless” suture-mediated patent foramen ovale (PFO) closure system.
Methods and results: Between June 2016 and October 2017, a prospective registry aimed at assessing the safety and efficacy of the NobleStitch EL (HeartStitch, Fountain Valley, CA, USA) suture-based PFO closure system was carried out at 12 sites in Italy. Among 200 consecutive patients evaluated, 192 were considered suitable for suture-mediated PFO closure (44±13 years, 114 female). Suture of the septum with the NobleStitch EL system was carried out successfully in 186 (96%) patients. Median fluoroscopy time was 16.1 (13.0-22.5) minutes and contrast volume 200 (150-270) ml. At 206±130 days follow-up, contrast transthoracic echocardiography with the Valsalva manoeuvre revealed no RLS (grade 0) in 139 (75%) patients and RLS grade ≤1 in 166 (89%) patients. Significant RLS was present in 20 (11%) patients (grade 2 and 3 in 11 and nine patients, respectively). There were no device-related complications.
Conclusions: The early results of this first Italian registry indicate that the suture-mediated “deviceless” closure of PFO is feasible in the majority of septal anatomies, and provides an effective closure of PFO comparable to traditional devices with a good safety profile at medium-term follow-up.
Abbreviations
ECG: electrocardiogram
PFO: patent foramen ovale
RLS: right-to-left shunt
RoPE: risk of peripheral embolism
TEE: transoesophageal echocardiography
TIA: transient ischaemic attack
TTE: transthoracic echocardiography
Introduction
In patients with paradoxical embolism through a patent foramen ovale (PFO) who are at increased risk of recurrent thromboembolic events, transcatheter closure of the atrial communication represents a more effective therapy than prolonged medical treatment1-3. Indeed, percutaneous PFO closure was shown to be safe and feasible with several prosthetic implantable occluder devices implementing different technologies based on an umbrella-like double disc design4-7. Despite the efficacy of PFO occluder devices, their use has a potential risk of early and late complications including, in extreme cases, device dislodgement, atrial wall erosion, perforation, fracture, migration-embolisation, infection, thrombosis, induction of arrhythmias and even death8-11. Additionally, the interatrial septum encumbrance of the prosthetic device may hinder future transseptal puncture and left-sided interventions such as left atrial appendage closure, arrhythmia ablation and mitral valve interventions. Finally, risk of allergic reactions to nickel mesh cannot be excluded, and the necessity of prolonged dual antiplatelet therapy after the procedure might not be tolerated by all patients12,13. Hence, a strategy of percutaneous PFO closure without a permanently implanted device represents an intuitive and revolutionary technique overcoming most of the limitations of traditional PFO occluders14. Recently, a new percutaneous “deviceless” system based on surgical suture-mediated PFO closure has been introduced in interventional practice. This study reports the first acute and early follow-up safety and efficacy results with this novel closure technique.
Methods
STUDY POPULATION
Between June 2016 and October 2017, a prospective multicentre observational registry aimed at assessing the safety and efficacy of a novel percutaneous suture-based PFO closure system (NobleStitch™ EL; HeartStitch, Inc., Fountain Valley, CA, USA) was carried out at 12 sites in Italy (Supplementary Appendix 1). Patients were eligible if they had a documented cryptogenic stroke or transient ischaemic attack (TIA), regardless of symptom duration, or if they had decompression sickness, intractable migraine and platypnoea-orthodeoxia associated with multiple ischaemic lesions at cerebral imaging and a PFO with atrial right-to-left shunt (RLS). Stroke and TIA were defined as previously described13. All patients underwent complete neurological and cardiologic examination, including brain computed tomography and/or magnetic resonance, 24-hour Holter-ECG monitoring, supra-aortic vessel Doppler ultrasound, and transthoracic (TTE) and/or transoesophageal (TEE) echocardiography. Exclusion criteria were the presence of even minimal aortic and/or carotid artery disease at carotid Doppler scan, TTE and/or TEE evidence of left-sided cardiac or aortic potential source of peripheral embolism, and evidence of repetitive supraventricular/ventricular rhythm disturbances at Holter-ECG monitoring. The Risk of Peripheral Embolism (RoPE) score was calculated for each patient15. As part of the standard selection protocol, all patients underwent a TTE and/or TEE microbubble test16. A detailed atrial septum anatomy description including atrial septal aneurysm (defined as abnormally redundant interatrial septum with an excursion of >10 mm into the right or left atrium and a base span of ≥15 mm) and an evaluation of microbubbles appearing in the left atrium during normal respiration and Valsalva manoeuvre were assessed in all patients16. Right-to-left shunt was semi-quantitatively graded according to the number of microbubbles detected in the left atrium after crossing the interatrial septum on a still frame within the first five cardiac cycles of contrast entering the right atrium. Grading was as follows: grade 0=none; grade 1 (minimal)=1 to 10 bubbles; grade 2 (moderate)=10 to 20 bubbles; grade 3 (severe) ≥20 bubbles16,17. Maximal RLS severity (occurring spontaneously or after Valsalva manoeuvre) was used for the analysis. Only patients with maximal RLS grade 2 or higher were included in the study.
CLOSURE PROCEDURE
The NobleStitch EL system consists of two dedicated suture delivery catheters (NobleStitch S and NobleStitch P) to capture and suture the septum secundum and the septum primum using a 4-0 polypropylene suture which produces an “S” shape closure of the PFO (Figure 1, Figure 2). The distal end of each NobleStitch has a suture-carrying arm which opens inside the heart to engage the septum correctly and an internal needle which pierces through the septum tissue picking the suture up in the opened suture-carrying arm. A third element, the KwiKnot™ catheter (HeartStitch, Inc.), is advanced over the septum secundum and septum primum sutures to approximate both septa, achieving closure by securing the stitch and trimming the excess suture material. For each patient, the right femoral vein was preferentially used to advance a multipurpose or similar catheter and cross the PFO with a 0.032” wire placed in most cases in the left upper pulmonary vein. After exchanging the multipurpose catheter for a straightened 14 Fr Mullins sheath and advancing a 0.018” wire into the distal superior vena cava or subclavian vein, sizing balloon interrogation of the PFO was performed to determine the anatomy of the septum secundum and septum primum during contrast injection (Figure 3A). The NobleStitch S and P were then sequentially advanced to suture the septum secundum and the septum primum, respectively, with special care taken to puncture the septum primum at the nadir of the septum secundum (Figure 3B-Figure 3E). Contrast injections were carried out according to operator discretion to obtain optimal engagement of each septum. After each NobleStitch needle had been advanced through the septum to capture and retrieve the suture, the system was removed along with the wire, leaving the sutures free. Then, the suture ends were gently pulled together to remodel the septum primum, drawing it towards the right atrium and closing the PFO. Maintaining the tension on the sutures, the KwiKnot delivery catheter was then used to advance and release a radiopaque polypropylene knot on the right side of the interatrial septum and to cut the proximal suture (Figure 3F). Contrast injection and TEE or TTE were performed in all patients to assess the acute result. All procedures were performed under fluoroscopic guidance providing direct visualisation with or without transoesophageal or intracardiac echocardiographic monitoring. Prophylactic antibiotics were administered in all patients and all patients were pre-treated with aspirin (100 mg daily). Patients received a minimum 70 IU/kg of heparin at the beginning of the procedure, followed by further boluses in order to maintain an activated clotting time constantly >250 seconds. After the procedure, antiplatelet therapy continuation was left to the discretion of the attending physician. In the absence of other indications, the standard protocol was aspirin 100 mg daily for one month. Antibiotic prophylaxis was advised for five days after discharge.
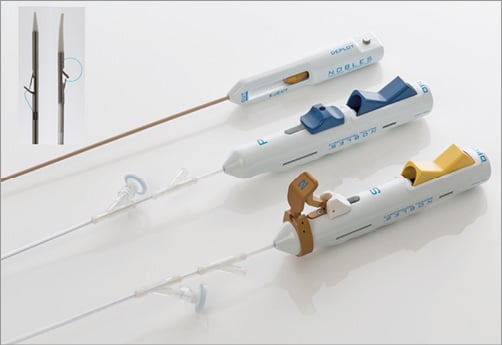
Figure 1. Description of the NobleStitch EL system. The NobleStitch EL suture delivery system consists of two dedicated catheters (NobleStitch S and NobleStitch P) to capture and suture the septum secundum and the septum primum using a 4-0 polypropylene suture. The distal end of each NobleStitch suture delivery has a suture-carrying arm which opens inside the heart to engage the septum secundum and primum and an internal needle which pierces through the septum tissue picking the suture up in the opened carrying arm. A third element, the KwiKnot catheter, is advanced over the septum secundum and septum primum sutures to secure the stitch and trim the excess suture material.
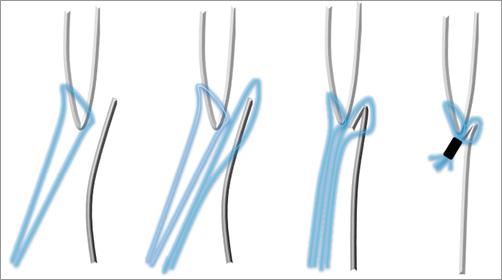
Figure 2. Schematic drawing showing the basic concept of the NobleStitch EL technique. A suture is sequentially pierced through the septum secundum and primum and then the two sutures are bound together.
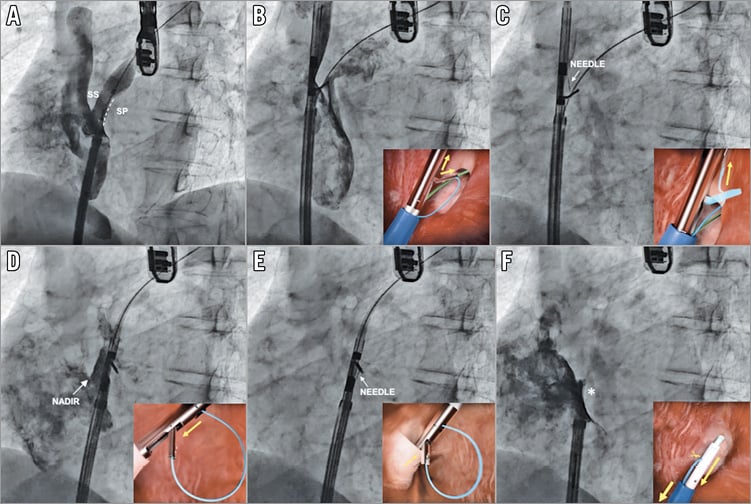
Figure 3. Percutaneous patent foramen ovale closure with the NobleStitch EL system. Sizing balloon interrogation of the PFO was performed to determine the anatomy of the septum secundum and septum primum during contrast injection (A). The NobleStitch S and P were then sequentially advanced to suture the septum secundum and the septum primum, with special care taken to puncture the septum primum at the nadir of the septum secundum (B-E). Contrast injections were carried out according to operator discretion to obtain optimal engagement of each septum. After each NobleStitch needle had fired, the delivery system was removed. Then, the suture ends were gently pulled to bend the septum primum towards the right atrium and close the PFO. Keeping the tension on the sutures, the KwiKnot delivery system was then used to advance and release a polypropylene knot at the right side of the interatrial septum (asterisk) and cut the proximal suture (F).
OUTCOME MEASURES AND FOLLOW-UP EVALUATION
Patients were followed up clinically at 1, 6 and 12 months after the procedure. The efficacy endpoint was effective PFO closure defined as residual RLS grade 1 or lower. The safety endpoint was a composite of adverse system- or procedure-related events including atrial fibrillation. A detailed list of adverse system-related and procedure-related events is provided in Supplementary Appendix 2. Major adverse events, including death, recurrent cryptogenic stroke or TIA, were individually recorded. A microbubble TTE was scheduled at 1-, 6- and 12-month follow-up, to evaluate residual RLS for the purpose of monitoring for change over time. To investigate the mechanisms of residual RLS further, all patients with significant residual shunt (grade 2 or higher) were carefully evaluated using TEE. Electrocardiographically documented arrhythmias (repetitive arrhythmias lasting >60 seconds) during a planned 24-hour Holter monitoring within three months after the endovascular procedure were also prospectively registered in symptomatic patients18. Procedure-related adverse events were carefully monitored, including serious bleeding requiring transfusions, groin haematoma (diameter >60 mm), any deep vein thrombosis, periprocedural arrhythmias, post-procedural serum creatinine increase (>20% above baseline level at 24 hours) and anaesthesia-/TEE-related events (Supplementary Appendix 2, Supplementary Appendix 3).
STATISTICAL ANALYSIS
To assess for possible differences between patients with and without significant RLS at follow-up, continuous variables were compared by Student’s t-test or Mann-Whitney U test as appropriate. Categorical variables were compared by Pearson chi-square test or Fisher’s exact test as appropriate. Data are reported as mean±standard deviation or as median (interquartile range), unless otherwise indicated. A two-sided p-value <0.05 was required for statistical significance.
Results
PATIENTS
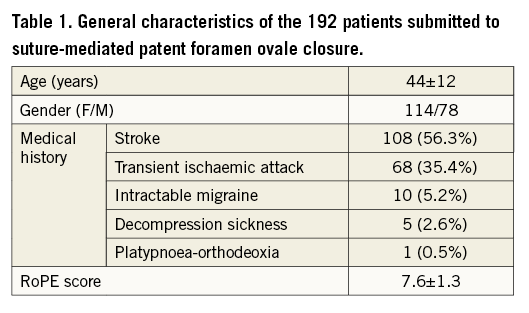
A cohort of 200 consecutive patients was evaluated for suture-based PFO closure. Eight patients were excluded after TEE and/or angiography because of atrial septal defect ≥10 mm (n=3), fenestrated PFO (n=4) and supraventricular tachycardia during induction of general anaesthesia (n=1). Overall, 192 patients (44±13 years, 114 female) were considered suitable for suture-mediated closure on the basis of the anatomical characteristics of the PFO (Figure 4). The general characteristics of the study population are reported in Table 1.
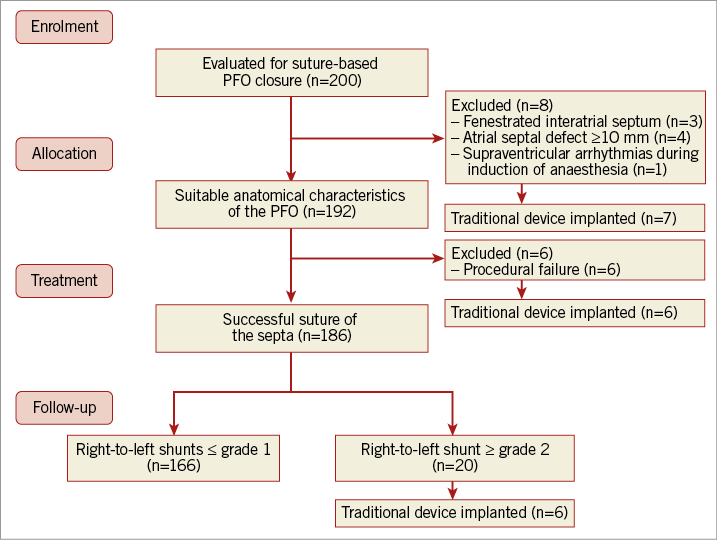
Figure 4. Flow chart of the study. Summary of patient enrolment, allocation, treatment, and follow-up.
PROCEDURAL OUTCOMES
Suture of the septum with the NobleStitch system was carried out successfully in 186 (96%) patients. In six (3.1%) patients, the procedure could not be completed due to technical difficulty in visualising interatrial septa (n=3) angiographically and/or the immediate result was not considered satisfactory because of unchanged basal shunt (n=3); in all cases an umbrella-like device was successfully implanted (Figure 4). In another 20 patients, an immediate RLS ≥grade 2 could be detected at TEE or TTE during the Valsalva manoeuvre. One patient developed palatal ecchymosis probably due to the TEE probe and another had a thrombus generated in the Mullins sheath prior to introduction of the NobleStitch S catheter which was completely aspirated without any consequence. Five patients developed a groin haematoma. In all cases, there were no clinical consequences. In 138 patients one set of delivery systems was used, in 44 patients two sets and in the remaining patients more than two sets of delivery systems were used. The need to use more than one set of devices was due to the failure to catch the septum secundum or primum properly at the first attempt. In two patients, two sutures were inserted due to the presence of significant residual shunt. In 86 (46%) patients, the procedure was carried out with fluoro-angiography monitoring only without echocardiographic support under local anaesthesia; in the remaining 100 (54%) patients, echocardiographic monitoring was used (TEE under general sedation in 83% or intracardiac echocardiography under local anaesthesia in 17%). Overall, 87% of planned echocardiographic guidance was performed in the first 100 patients and 13% in the second 100 patients. The median duration of the procedure was 58 (40-75) minutes and median contrast medium used was 200 (150-270) ml. The median radiation dose absorbed per patient was 87 (52-125) Gy·cm2. Ninety-three percent of the patients were discharged asymptomatic and in good condition within 24 hours after the procedure and all within 48 hours.
FOLLOW-UP
The mean follow-up of the 186 patients who completed the procedure was 206±130 days. The functional, anatomical and procedural characteristics are reported in Table 2. Overall, 42 (22.6%) patients completed the 12-month follow-up and 116 (63.4%) patients completed the six-month follow-up. No recurrent cerebral events or any other clinical sequel were recorded during follow-up; Holter-ECG monitoring carried out in 36 symptomatic patients resulted in being negative for repetitive arrhythmias lasting >60 seconds and no patient had atrial fibrillation. At maximal follow-up, TTE evaluation showed a complete closure (RLS grade 0) in 139 (75%) patients and an RLS grade 1 or lower in 166 (89%). Significant RLS was present in 20 (11%) patients (grade 2 and 3 in 11 and nine patients, respectively) (Figure 5). In six patients, an umbrella device was implanted, while in the remaining patients, all asymptomatic, the clinical decision was to wait until the 12-month follow-up to verify the possibility of late closure. Of note, no changes in the closure rate on TTE were observed at different follow-up timings, suggesting the maintenance of the suture without late untying.
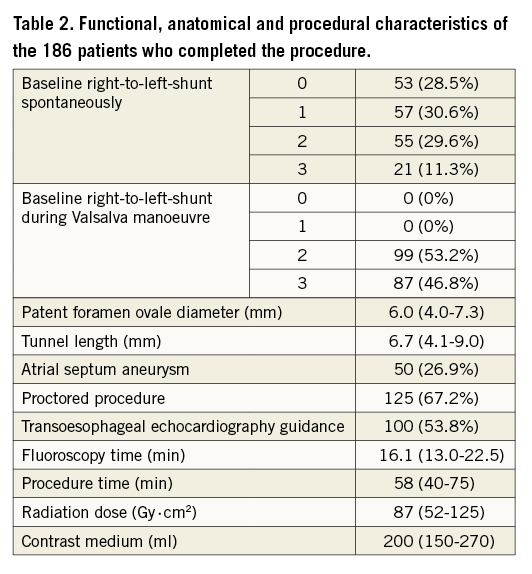
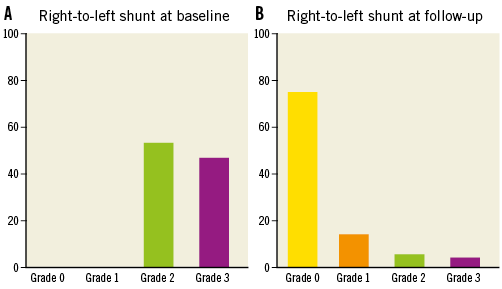
Figure 5. Right-to-left shunt. Results of contrast transthoracic echocardiography during the Valsalva manoeuvre at baseline (A) and at follow-up (B).
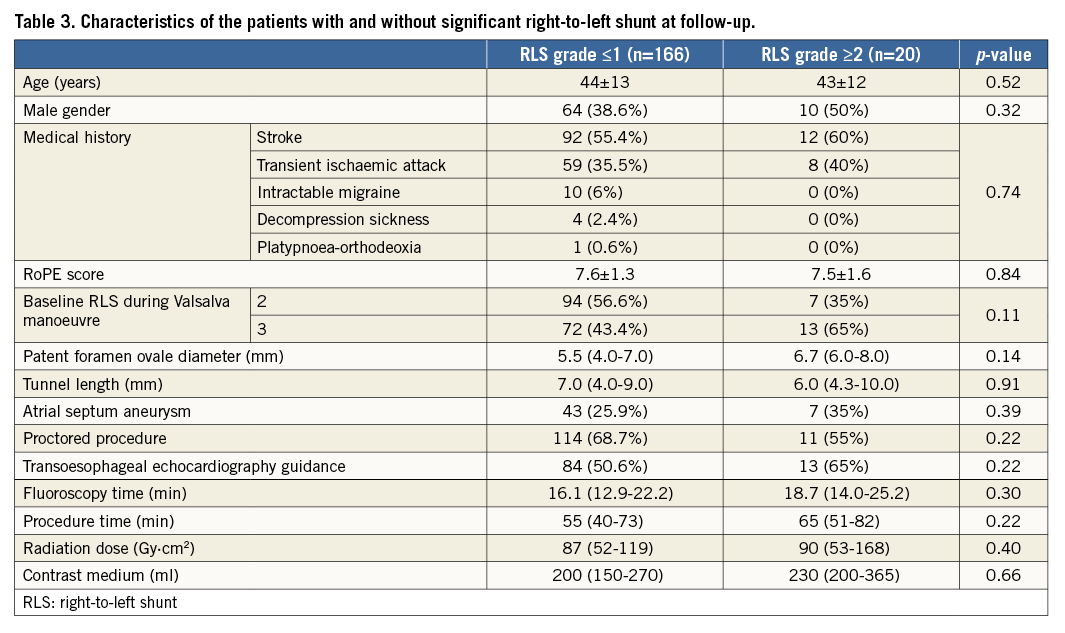
Among patients with a significant residual RLS, a fenestration simulating a small atrial septal defect which was probably undetected prior to suturing of the septum primum was detected at one-month follow-up in three patients. In four patients, an asymmetrical positioning of the suture, situated more anteriorly towards the aortic rim, was considered the probable cause of a wide septal opening in the posterior site of the septum causing residual shunt. In the remaining patients, a reasonable cause of residual RLS could not be identified as both sutures and knot appeared to be well positioned. In one asymptomatic patient, at six-month TTE follow-up, showing no residual RLS, a round thrombus localised in the right atrium and apparently connected to the Eustachian valve was visualised. This patient, suffering from pre-existing inherited thrombophilic disorder with a history of repeated thrombi formation in the venous system and spontaneous abortion, was treated with oral anticoagulation. There were no recorded suture-related complications. No clinical or procedural variable was significantly associated with residual RLS at follow-up (Table 3).
Discussion
The findings of this multicentre registry indicate that suture-mediated PFO closure represents a valid and revolutionary alternative to traditional umbrella-like devices. The initial experience of the Italian registry indicates that the technique is feasible and safe in the majority of septal anatomies and provides an effective closure of PFO comparable to traditional devices. In the recent Gore REDUCE Study (HELEX® Septal Occluder and CARDIOFORM Septal Occluder; both W.L. Gore & Associates, Flagstaff, AZ, USA) the complete closure rate at 12 months was 75.6% and the effective closure rate defined as “freedom from large shunt (>25 bubbles) in subjects with retained study device as detected by transthoracic echocardiography adjudicated by echo core lab” was 94.5%3. In the RESPECT trial (AMPLATZER™ PFO occluder; St. Jude Medical, St. Paul, MN, USA), CLOSURE 1 trial (STARFlex® occluder; NMT Medical Inc., Boston, MA, USA), and the PC trial (AMPLATZER PFO occluder), a slightly different criterion for effective closure was used, defining it as a shunt grade of 1 or lower19-21. By using this definition, in the RESPECT trial an effective closure rate of 93.5% and a full closure rate of 72.7% was achieved19, while in the CLOSURE 1 trial an effective closure rate of 86.7% was reported20. The PC trial achieved an effective closure rate of 95.9%21. Using this definition of effective closure, in the present registry the NobleStitch EL system achieved a closure rate of 89% and a full closure rate of 75% that is comparable with traditional devices. This is remarkable considering the initial learning curve needed to achieve technical competence. This latter issue should be underscored when a novel technique is compared to a consolidated and well-standardised procedure introduced 20 years ago. It demonstrates that procedural skill can be achieved in a relatively short period of time. Of note, regarding the procedure, although the suture-mediated PFO closure technique, compared to the traditional system, requires a particular meticulousness and probably an additional amount of contrast medium and radiation dose, the lack of the need for general sedation and echocardiographic monitoring largely compensates for these drawbacks. Indeed, echocardiography is frequently used to guide percutaneous PFO closure procedures in order to optimise device selection and implantation; hence, it appeared reasonable that, initially, for the early experience of a novel technique, echocardiographic monitoring support was used in each centre. However, growing experience with the NobleStitch system has shown that echocardiography guidance for percutaneous PFO closure is not necessary in the majority of cases provided that an accurate preprocedural echocardiographic evaluation is carried out. Most importantly, the safety profile is a very important aspect of this novel technique. Indeed, while the rates of the two PFO closure approaches are comparable, the device/procedure safety data of the present registry deviate significantly from previous published reports in which an umbrella-like occluder was used, as no major technique- or device-related adverse effects were recorded. This contrasts with the rates of major adverse events found in the CLOSURE I trial (16.9%), RESPECT trial (4.2%), Gore REDUCE Study (12.8%), CLOSE trial (5.9%) and the PC trial (21.1%)1,3,19-21. Another important issue relating to this novel PFO closure technique is that, in the case of failure, this approach does not preclude the possibility of implanting an umbrella device as no permanent metallic insert remains in the heart.
In the attempt to define determinants of residual RLS aimed at selecting patients with the most favourable anatomies for a suture-mediated closure technique, we could not find any variable associated with residual RLS. Indeed, the careful analysis of all patients with residual RLS suggests that, in the presence of a wide redundant septum, particularly the septum primum, a single suture might not be sufficient to approximate both septa to close the PFO completely. Further experience with the technique is needed to investigate this issue more deeply, including the ability to place additional sutures to achieve closure in this group.
Limitations
This study presents a number of limitations that should be acknowledged. This is the very first systematic multicentre report on a novel technique that is not yet fully standardised, so there was a rather wide variability in some procedural variables such as the amount of contrast medium used, procedure duration and the X-ray dose absorbed per patient. All the procedures were proctored by experienced operators (A. Gaspardone, F. De Marco, M. Mullen) so the results obtained in this registry might not be achieved in the case of less experience with the devices and technique. Additionally, a central core laboratory for angiographic and echocardiographic evaluation was not available so all data were validated by each individual centre. Finally, the duration of the follow-up was relatively short so late opening due to suture release cannot be totally excluded; however, such an eventuality did not occur in any of the patients with one-year follow-up.
Conclusions
The early results of this first Italian registry indicate that the suture-mediated “deviceless” closure of PFO is feasible in the majority of septal anatomies, and provides an effective closure of PFO comparable to traditional devices with a good safety profile at medium-term follow-up. If these preliminary results are confirmed in larger series, this technique, overcoming most of the limitations of the traditional PFO occluders, may represent the first choice technique to close PFO.
| Impact on daily practice Transcatheter closure of patent foramen ovale (PFO) in patients with paradoxical embolism represents a more effective treatment than prolonged medical therapy. However, traditional devices used for transcatheter PFO closure are associated with risks and limitations including, in extreme cases, embolisation, erosion, thrombus, arrhythmias, hindered access to left atrial structures and even death. Against this background, the results of our study show that a novel suture-mediated “deviceless” PFO closure system is feasible in the majority of septal anatomies and provides an effective closure of PFO comparable to traditional devices with a good safety profile at medium-term follow-up. |
Conflict of interest statement
A. Nobles is the inventor of the NobleStitch EL suture delivery system, and is a paid officer and shareholder of Nobles Medical Technologies II (NMT2) and HeartStitch, Inc. M. Mullen is a paid consultant of HeartStitch, Inc. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. The institutions and investigators that participated in the NobleStitch EL Italian Registry.
Supplementary Appendix 2. Definition of complications related to NobleStitch EL endovascular treatment.
Supplementary Appendix 3. Sample size calculation.
To read the full content of this article, please download the PDF.

