
Abstract
Aims: The aim of this observational study was to compare acute and 12-month results of percutaneous closure of patent foramen ovale (PFO) with two occluder devices.
Methods and results: Between June 2007 and October 2014, 406 consecutive patients (48.1±13.3 years, 243 women) underwent percutaneous PFO closure with either the AMPLATZER (n=179) or the Figulla (n=227) device after a stroke or a transient ischaemic attack ascribed to the PFO. A right-to-left shunt grade >1 was previously detected in all patients and atrial septal aneurysm was present in 111 (27.5%) patients. Patients were followed up with a contrast transthoracic echocardiogram and clinically at 24 hours, six months, and 12 months after the procedure. A high procedural success was observed in both groups. Despite a trend towards a higher incidence of acute residual shunt immediately after device deployment among Figulla occluder patients, a residual grade ≥2 right-to-left shunt was observed in 4.5% of patients, independently of the device used for PFO closure. The only difference reported after Figulla device implantation was a lower rate of supraventricular arrhythmias (9% vs. 17%, p=0.02).
Conclusions: According to this two-centre study, PFO closure appears safe and effective with the Figulla occluder as well as with the AMPLATZER device.
Introduction
In patients with presumed paradoxical embolism through a patent foramen ovale (PFO) who are at increased risk of recurrent thromboembolic events, transcatheter closure of the atrial communication represents an alternative to lifelong medical treatment. Percutaneous PFO closure has been shown to be safe and feasible with several occluder devices implementing different technologies based on an umbrella, a disc or a bioabsorbable design1-6. Several studies have previously compared the performance of PFO occluders based on markedly different fabric7-10. Whether slight differences in the structure and design of the occluder might have a significant impact on the outcomes related to the procedure is unknown. Therefore, the aim of this study was to compare procedural and clinical results up to one year of the two most diffuse nitinol PFO closure devices, which differ in their braiding techniques and by the quantity of the meshwork material present on the left atrial side.
Methods
An observational registry was run in two Italian centres (Milan and Rome) to recruit all consecutive patients treated with either the AMPLATZER™ PFO Occluder (n=179) (St. Jude Medical, St. Paul, MN, USA) or the Occlutech® Figulla® device (n=227) (Occlutech GmbH, Jena, Germany) between June 2007 and October 2014.
SCREENING PROTOCOL AND DEFINITIONS
A careful screening protocol including accurate clinical history, transthoracic echocardiography (TTE), coagulation analysis and a complete laboratory screening for thrombophilia (antithrombin III, anticardiolipin, antiphospholipid antibodies, lupus anticoagulant, protein C and S, homocysteine; genetic tests for factor V Leiden and factor II) was carried out in all enrolled patients. Thrombophilia was defined by ≥1 abnormal test result. A brain magnetic resonance (MR) or computed tomography (CT) scan was routinely performed in all patients. All patients underwent transoesophageal echocardiography (TEE) prior to the PFO closure procedure. Right-to-left-shunt (RLS) was semi-quantitatively graded according to the number of microbubbles detected in the left atrium after crossing the interatrial septum on a still frame during the first five cardiac cycles of contrast entering the right atrium. Grading was as follows: Grade 0: no bubbles; Grade 1 (trivial): <10 scattered bubbles seen in the left heart; Grade 2 (moderate): obvious shunt with >10 bubbles seen in the left heart; Grade 3 (large): >20 bubbles with partial or complete opacification of the left heart11. Maximal RLS severity was used for the analysis. Only patients with RLS >1 were enrolled. All echocardiographic pre- and post-closure examinations were reviewed by two independent experts blinded to the device used. The criteria for atrial septal aneurysm were a diameter of the base ≥15 mm and a total excursion of the septum ≥10 mm12. Moreover, according to our centres’ protocol, all patients underwent arrhythmia screening with 24-hour Holter monitoring, before indication to PFO closure.
PFO CLOSURE DEVICES
The AMPLATZER PFO Occluder is a self-expanding double-disc device composed of a 0.005-inch nitinol wire with a polyester fabric patch sewn into both discs (Figure 1). The device has a flexible and stretchable 3 mm waist and one hub on each disc. The PFO Occluder is available in sizes (left atrial side) 18, 25, 30 and 35 mm.
The Occlutech Figulla device consists of a single layer nitinol wire mesh forming two flexible retention discs (2 mm diameter smaller on the left side) with a hub on the right side only (Figure 1). The discs are connected by a flexible and stretchable 3 mm waist in the centre. The left atrial disc is a single flat layer covered by an ultrathin polyethylene terephthalate patch. The size of the Figulla device is determined by the diameter of the two discs with the following available configurations: 16/18, 23/25, 27/30 and 31/35 mm.
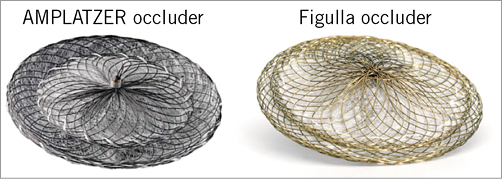
Figure 1. Graphical representation of the AMPLATZER PFO Occluder and the Occlutech Figulla device, differing in terms of their braiding techniques and by the quantity of the meshwork material present on the left atrial side.
PROCEDURAL PROTOCOL
Transcatheter PFO closure was performed by standard technique according to the manufacturer’s instructions. Under echocardiographic guidance, allowing a careful assessment of the fossa ovalis anatomy and the presence or absence of atrial septum aneurysm, a 0035” J-tipped guidewire was positioned through the atrial septum into the upper pulmonary vein. Intracardiac ultrasound guidance (Ultra ICE™; Boston Scientific, Marlborough, MA, USA), avoiding patient intubation, was the procedural guiding strategy used in Milan, while all PFO closures carried out in Rome were performed with patients in light sedation under fluoroscopic and TEE monitoring.
In all cases, an appropriately sized occluder was loaded into a long 8-10 Fr introducer sheath and advanced by pushing the delivery cable to the tip of the sheath positioned in the left atrium. The choice of occluder depended on the alternating availability of the devices and physician preference prior to the procedure. Owing to a policy ensuring a per patient availability of PFO occluders, the interventional cardiologist on duty for PFO closure had to request occluder availability for the day of the procedure. The selection was unrelated to the patient and it was never possible to choose another device type during the procedure. Under fluoroscopic guidance in a left anterior oblique projection and echo guidance, the left atrial disc was deployed and pulled back gently against the atrial septum. Using gentle tension on the delivery cable, the sheath was pulled back and pushed forward and the right atrial disc was deployed. Ultrasound evaluation was performed after device deployment to check the adequate positioning of the device, possible obstruction to systemic or pulmonary venous return and impairment of the atrioventricular valves. The device was then released.
MEDICATION PROTOCOL
Patients received heparin 70 IU/kg at the beginning of the procedure followed by further boluses in order to maintain an activated clotting time >200 seconds. Aspirin (100 mg/day) was started at least 24 hours before the procedure and continued for six months after PFO closure, while clopidogrel was administered immediately after the procedure and continued for three months. Antibiotic prophylaxis was given before the procedure and then for five days.
FOLLOW-UP AND ENDPOINT DEFINITION
To assess possible differences in relatively early events, all patients were followed up clinically at one, six and 12 months after the procedure. Major adverse events, including death, cryptogenic stroke or transient ischaemic attack (TIA) were individually recorded. Cryptogenic stroke was defined as a clinical syndrome consisting of focal or global neurologic deficit, associated with a related lesion on a CT or MR scan, that had no known underlying cause despite a thorough evaluation with currently available diagnostic procedures. Cryptogenic TIA was defined as a clinical syndrome consisting of a transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischaemia without acute infarction as assessed by CT or MR scan13. Electrocardiographically documented arrhythmias during a planned Holter monitoring at one-month follow-up were also prospectively registered. A contrast TTE was performed within 24 hours and at six-month follow-up, and blindly evaluated by two independent operators, to assess PFO occluder position and evaluate residual RLS (inter-observer difference <0.5%).
STATISTICAL ANALYSIS
Continuous data with a normal distribution, according to the Kolmogorov-Smirnov test, are reported as means with standard deviations and were compared with the Student’s t-test. Data with a non-normal distribution are reported as median (interquartile range) and were compared with the Mann-Whitney U test. Categorical data were compared by Pearson’s chi-square test or Fisher’s exact test as appropriate, and are presented as frequencies and/or percentages. Inter-observer agreement was assessed by the Pearson’s correlation test. A two-sided p-value ≤0.05 was required for statistical significance.
We have waived an a priori sample size calculation because of the difficulties of building up such an estimation owing to the similarity of the two devices and the generally low rate of adverse events after transcatheter PFO closure.
Results
BASELINE
A flow chart of the study is shown in Figure 2. Overall, 406 patients were enrolled (48±13.3 years, 243 women). An AMPLATZER occluder was implanted in 179 patients and a Figulla device was implanted in the remaining 227 patients (Figure 3). Their baseline characteristics are listed in Table 1. The groups were similar in terms of age, gender, interatrial shunting grade and septal anatomy, clinical indication to PFO closure, device size and hospital site. There were no differences concerning cardiovascular risk factors except for a higher incidence of hypertension and smoking habit among patients treated with an AMPLATZER occluder device. A previous clinical ischaemic stroke was present in 34% and a transient ischaemic attack in 66% of the patients. Anatomic evaluation of the fossa ovalis showed coexisting septal atrial aneurysm in 111 (27.5%) patients.
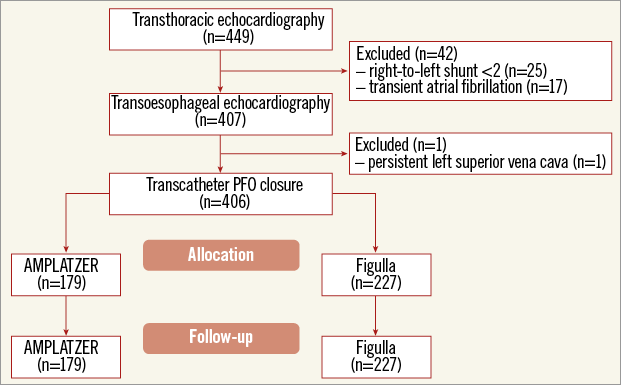
Figure 2. Flow chart of the study. Initially, 449 patients with a clinical history potentially consistent with patent foramen ovale (PFO) were screened by transthoracic echocardiography. Of these, 42 were excluded because of an excessively mild right-to-left shunt (n=25) or because of an electrocardiographic demonstration of episodes of atrial fibrillation (n=17). After TEE to confirm the diagnosis of PFO, one more patient was excluded because of persistent superior vena cava. Overall, 406 patients underwent transcatheter PFO closure, 179 with the AMPLATZER PFO Occluder and 227 with the Occlutech Figulla device.
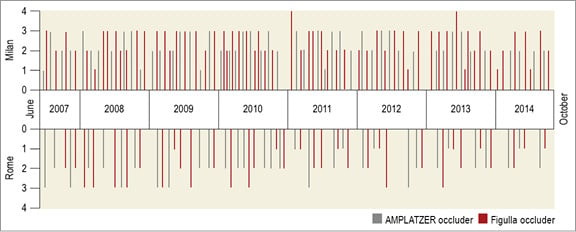
Figure 3. Time distribution in each centre of the transcatheter patent foramen ovale closure procedures using either the AMPLATZER occluder or the Figulla device showing the balance between groups and centres and over time. Overall, 406 patients underwent transcatheter patent foramen ovale closure with the study devices between June 2007 and October 2014. An AMPLATZER occluder or a Figulla device was variably used in 111 and 155 patients, respectively, in Milan, and in in 68 and 72 patients, respectively, in Rome.
PROCEDURE OUTCOME
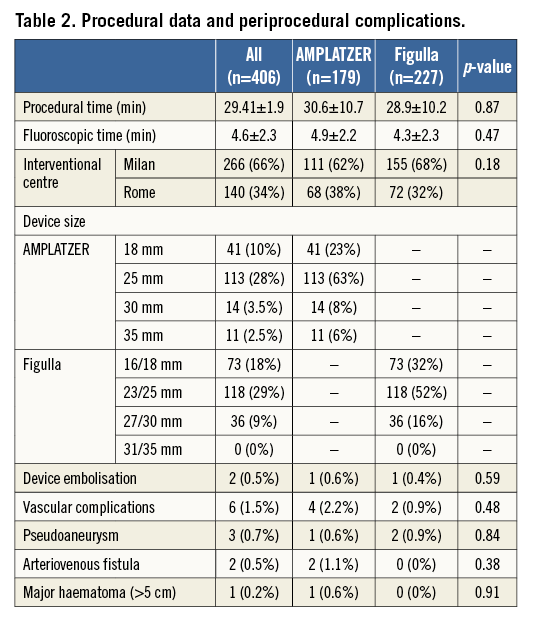
Cumulative procedural success was 99.5%; there was a 25 mm AMPLATZER device late displacement and a 16/18 mm Figulla embolisation, successfully managed via percutaneous device retrieval. Device embolisation occurred in both cases in a patient with a tunnel-like PFO and incomplete alignment of the device discs to the atrial septum after implantation, thus favouring occluder dislocation and embolisation. Periprocedural complications are reported in Table 2. Notably, no ischaemic events occurred during or after the procedure.
ECHOCARDIOGRAPHIC FOLLOW-UP
Overall agreement between echocardiographers was rather high (r=0.94). A contrast TTE was performed in all patients within 24 hours after the procedure showing residual shunting in 89 (22%) patients overall, 43 (24%) in the AMPLATZER group and 46 (20%) in the Figulla group (Figure 4). All devices were correctly positioned and no thrombi were detected. Six-month contrast TTE was performed in all patients and showed a reduction of shunting and complete closure in 26 out of the 43 AMPLATZER patients and in 27 out of the 46 Figulla patients (p=0.96). Residual shunts at six months were Grade 1 in nine (5%) AMPLATZER patients and in nine (4%) Figulla patients and Grade 2 in eight (4.5%) AMPLATZER patients and 10 (4.5%) Figulla patients (Figure 4). Table 3 shows baseline and procedural data in patients with and without residual left-to-right shunt at six-month follow-up. The two groups of patients did not differ significantly according to any of the explored variables. Patients with residual shunts at six months continued aspirin treatment and were scheduled for yearly follow-up thereafter.
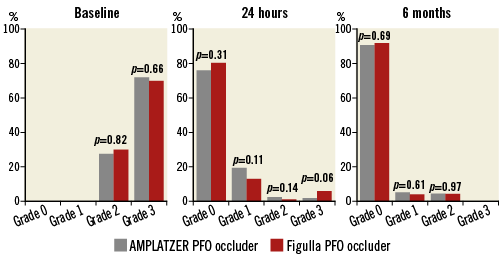
Figure 4. Comparison of right-to-left shunt as assessed by echocardiography in the two study groups at baseline, 24-hour and six-month follow-up following transcatheter patent foramen ovale closure. At baseline, all patients had a Grade 2 or 3 shunt. At 24 hours, different degrees of residual shunting were found in 89 (22%) patients overall, 43 (24%) in the AMPLATZER group and 46 (20%) in the Figulla group. At six-month follow-up, echocardiography showed a reduction of shunting and complete closure in 26 out of the 43 AMPLATZER patients and in 27 out of the 46 Figulla patients (p=0.96). No patients had Grade 3 shunt anymore. Residual shunts at six months were Grade 1 in nine (5%) AMPLATZER patients and in nine (4%) Figulla patients (p=0.61) and Grade 2 in eight (4.5%) AMPLATZER patients and 10 (4.5%) Figulla patients (p=0.97).
CLINICAL FOLLOW-UP
No patient was lost to follow-up. No death, recurrent embolic event or aortic erosion occurred during follow-up (Table 4). Atrial fibrillation was observed in four AMPLATZER patients and one Figulla patient immediately after device deployment or during the first 30 days; accordingly, supraventricular arrhythmias were more frequently observed in the AMPLATZER group (17%) compared to the Figulla group (9%) (p=0.02). Two thirds of arrhythmic events were periprocedural or occurred during hospital stay, whereas the remaining were observed during the one-month Holter monitoring. In all cases, arrhythmias were resolved by pharmacological treatment.
Discussion
This study is the largest clinical and contrast-enhanced echocardiography comparative assessment of two similar devices for transcatheter PFO closure, namely the AMPLATZER and Figulla occluders, with a 12-month follow-up. Our results show that high procedural success with a very low complication rate may be achieved using both these devices.
Despite the value of PFO percutaneous treatment for the prevention of thromboembolic events in patients with cryptogenic stroke14,15, some procedural and device-related complications may occur. Newer devices, with a lower left disc metallic mass and easier deployment and retrieval have been designed and tested, each with clinical and technical advantages and disadvantages.
The Figulla device has been designed with these specific novel technological properties, giving the device lower metallic mesh on the left atrial side and higher flexibility, allowing ideal septal alignment16. Krizanic et al showed good biocompatibility with rapid and complete neoendothelialisation in a swine model, and the first clinical trial by the same group showed the feasibility and safety of transcatheter PFO closure with the Figulla device4,17. A previous single-centre case control study by Saguner et al reported, in a small sample comparison between Figulla (n=20) and AMPLATZER (n=20) devices in PFO treatment, a 15% incidence of procedural complications in the Figulla group18. In particular, the Figulla PFO device was associated with a higher residual shunt incidence at six months compared to the AMPLATZER (39% vs. 0%). However, these data were not confirmed by Aytemir et al, who evaluated early to midterm results after Figulla device implantation in 85 patients with PFO and in 58 patients with atrial septal defect closure, showing no residual shunt in the PFO group19. In another similar study by Van den Branden et al, among 82 consecutive patients who underwent PFO (n=48) and atrial septal defect (n=34) closure, the reported procedural success was 100% with a low complication rate20. Previous studies comparing rather distinct PFO devices differ in assessed endpoints and follow-up duration, thus allowing limited comparison with our study7-10.
In a previous study, Pac et al reported similar procedural success and complication rates between Figulla and AMPLATZER occluders in a single-centre study enrolling 75 consecutive patients with atrial septal defect21. Additionally, they reported more periprocedual residual shunt that disappeared after six months. Percutaneous PFO closure has a success rate close to 100%, eliminating the RLS shunt in 90% of patients1,22,23. However, the presence of moderate to severe residual shunts has been described in 2-10% of patients1,24,25 and related to an inadequate design of the device, multiple septal fenestration or interatrial septal aneurysm. In our series, significant residual shunt following PFO closure was observed in 4% of all patients, occurring equally among the AMPLATZER and Figulla device groups. The clinical importance of these residual shunts has been previously elucidated and, while small residual shunts do not appear to have clinical significance26, the presence of more than a moderate grade shunt after PFO closure appears to be related to increased risk of recurrent stroke during the follow-up2. Grade 1 shunts during Valsalva manoeuvre immediately after closure device implantation resolved spontaneously during the follow-up period in 75% and 70% of cases in the AMPLATZER and the Figulla group, respectively. Similarly, grade ≥2 shunts showed a 35% reduction in the Figulla group but no changes in the AMPLATZER group. We believe that the progressive reduction of RLS severity and its resolution over time after Figulla device deployment might be explained by differences in design between the two devices, with the Figulla occluder requiring a more complete endothelialisation process to avoid shunting from the right to left atrium. Notably, according to our data, none of the patients or procedural characteristics seems to help identify those at risk of having a residual shunt.
The increased prevalence of supraventricular arrhythmias observed among AMPLATZER patients may likewise be explained by the differences in design between the two devices or may simply be due to chance. Certainly, Holter monitoring is an inherently limited method for arrhythmia assessment but there is no other practical or ethical way to assess real-world PFO patients without specific risk factors or clues pointing towards supraventricular arrhythmias. Moreover, it has been previously shown that arrhythmias represent a constitutional feature of the PFO syndrome27 and that PFO closure using different occluder devices does not appear per se to be an inductor of post-procedural arrhythmias28.
Limitations
Lack of randomisation represents the main limitation of this study. However, the choice of the device was unsystematic being due to the alternating availability of the devices and physician preference prior to the procedure. Additionally, despite the fact that the main baseline characteristics were substantially comparable in the two groups of patients, hypertension and active smoking were more frequent in the AMPLATZER group. The duration of follow-up was relatively short to assess any differences in events with a low rate of occurrence such as major adverse clinical events. Differences in supraventricular arrhythmias between the two groups were found on a single Holter monitoring at one month and it cannot be excluded that a longer follow-up would provide different findings. Moreover, TEE would possibly have improved the detection of residual RLS, although with higher costs, higher complexity and higher risks as trade-off. However, our study was exploratory and not specifically directed to the assessment of residual shunt; TEE may not be superior to TTE in detecting RLS due to difficulties in performing an appropriate Valsalva manoeuvre. Among the several anatomic features that have been associated with PFO outcomes in cryptogenic stroke, our study only reports on atrial septal aneurysm and atrial septal bulging. However, there is currently no evidence that a specific high-risk anatomic feature excludes the role of PFO in cryptogenic stroke. On the contrary, in such circumstances PFO closure is even more compelling. However, it should be acknowledged that the unmatched prevalence among the study groups of high-risk anatomic characteristics other than those reported may unevenly increase the risk of events, especially in the long term. Finally, lack of an a priori sample size calculation is another limitation of our study, as previously stated.
Conclusions
In our study, transcatheter percutaneous PFO closure was achieved safely and effectively with the AMPLATZER and the Figulla devices. Acute procedural success was high. Clinical results at 12-month follow-up were comparable between the two groups, and no relevant differences in residual shunt were found as evaluated by contrast TTE.
| Impact on daily practice In patients with presumed paradoxical embolism through a patent foramen ovale (PFO) who are at increased risk of recurrent thromboembolic events, transcatheter closure of the atrial communication represents a valid alternative to lifelong medical treatment. Percutaneous PFO closure has been shown to be safe and effective with several occluders based on different technologies. We compared the two most used nitinol PFO closure devices, which differ by their braiding techniques and quantity of the meshwork material present. Good results in terms of safety and efficacy achieved with both the AMPLATZER and the Figulla occluders suggest that differences in the structure and design of the devices do not have a significant impact on clinical success. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

