
Abstract
Aims: Residual shunt following percutaneous patent foramen ovale (PFO) closure has been described in up to 49% of patients and is associated with recurrent cerebrovascular events. Our aim was to evaluate the safety, feasibility, and midterm outcomes of transcatheter residual shunt closure.
Methods and results: From 1994 to July 2016, 2,679 patients underwent transcatheter PFO closure for treatment of presumed paradoxical embolism at our institution. Among them, 100 patients (3.7%) were referred for residual shunt closure. They constituted the study population for which a retrospective analysis of the prospectively gathered procedural data was performed along with prospective acquisition of follow-up data. The indication for initial PFO closure was an ischaemic cerebrovascular event in 85% of the patients. Patients underwent transoesophageal echocardiography (TOE) for PFO diagnosis and again for residual shunt assessment at about six months. All procedures were performed under fluoroscopic guidance only. At the first procedure, 10 different devices had been used. The AMPLATZER PFO Occluder accounted for 54% and the AMPLATZER Cribriform Occluder for 28%. Compared to the whole population (n=2,679), a significantly higher rate of atrial septal aneurysm (58% versus 36%; p=0.024), a larger proportion of shunt grade 3 at baseline (97% versus 78%; p<0.001), and a larger size (≥30 mm) of the first implanted device (47% versus 13%; p<0.001) were observed in the patients with residual shunt. Six patients (6%) experienced a recurrent TIA or ischaemic stroke before the second intervention. Residual shunt closure was successful in all but two patients. A second AMPLATZER PFO Occluder was used in the majority of the repeat interventions (76%). There were no complications. TOE, obtained again after 7±5 months in 88 of the 98 patients with a device in place (90%), showed complete closure in 81%. In eight patients (0.3% of the whole cohort), a third device was implanted, resulting in complete closure in all.
Conclusions: Transcatheter residual shunt closure after initial percutaneous PFO closure can be safely performed under fluoroscopic guidance only and achieves complete closure in most patients. The use of larger devices, typically prompted by intricate anatomy, represents a risk factor for shunt persistence and the need for reintervention.
Abbreviations
ASA: atrial septal aneurysm
ASD: atrial septal defect
PFO: patent foramen ovale
TIA: transient ischaemic attack
TOE: transoesophageal echocardiography
TTE: transthoracic echocardiography
Introduction
The association of a patent foramen ovale (PFO) with cryptogenic stroke, first reported independently by Lechat et al1, and Webster et al2 in 1988, has been repeatedly confirmed in older patients (>55 years)3-5. Under medical treatment alone, patients with cryptogenic stroke related to PFO are at risk of recurrence, with yearly stroke rates reported to be from 0.6 to 12.0%6,7. The risk is particularly pronounced in patients with associated atrial septal aneurysm (ASA)6,8. Numerous observational studies have suggested the superiority of percutaneous PFO closure over medical treatment for secondary prevention of paradoxical embolism7,9-11. One even showed a mortality benefit of PFO closure at 10 years of follow-up11. However, the superiority of PFO closure failed to be unequivocally confirmed in three randomised trials published to date12-14. Importantly, all three trials numerically supported closure but presented methodical flaws potentially impacting on their ability to discern differences effectively15. While the intention-to-treat analysis of the largest trial did not reach statistical significance, the as-treated analysis supported better outcome in the patients of the closure group14. In the first of these trials (CLOSURE: Evaluation of the STARFlex septal closure system in patients with a stroke and/or transient ischaemic attack due to presumed paradoxical embolism through a patent foramen ovale), a high rate of residual shunting (24%) was reported using the since abandoned STARFlex occluder device. This may have negatively impacted on the clinical outcomes of the patients included in the intervention group12.
Overall, residual shunt following percutaneous PFO closure has been described in up to half of the patients with strong variability according to the type of device used16. Moderate to severe residual shunting after attempted PFO closure has been related to an increased risk of recurrence17. However, data specifically analysing repeat intervention are limited18-23. The aim of the present study was to investigate the safety, feasibility, and midterm outcome of repeat transcatheter closure as a management strategy for significant residual shunts.
Methods
PATIENTS
From 1994 to July 2016, 2,679 patients underwent transcatheter PFO closure for treatment of presumed paradoxical embolism at our institution. Spontaneous or provoked right-to-left shunt was semi-quantitatively graded according to the number of bubbles crossing the interatrial septum on a single frame at transoesophageal echocardiography (TOE): grade 0=none, grade 1=minimal (1-5 bubbles), grade 2=moderate (6-20 bubbles), and grade 3=severe (>20 bubbles)2. Residual shunt grades 1, 2, and 3 were diagnosed in 7%, 3%, and 2%, respectively. Among the patients with residual shunt grade ≥2 (5%), 100 patients (3.7%) were referred for residual shunt closure and constituted the study population. After informed patient consent, data were prospectively collected and entered into a dedicated databank. For this subgroup study, a retrospective analysis of the prospectively gathered procedural data was performed along with prospective acquisition of follow-up data.
Paradoxical embolism was assumed in patients fulfilling the following criteria: (1) the presence of a PFO detected on TOE, (2) radiologically or clinically confirmed ischaemic stroke, transient ischaemic attack (TIA), or peripheral embolism (including myocardial infarction), and (3) exclusion of any concurrent cardiac, aortic, or cerebrovascular causes during standardised work-up encompassing colour-coded Duplex ultrasound of the extracranial and intracranial arteries, TOE, and 24-hour electrocardiography.
ECHOCARDIOGRAPHY
The diagnosis of PFO, ASA, and residual shunt after about six months of follow-up was based on contrast TOE performed under conscious sedation. An aerated colloid solution was injected into an antecubital vein at the end of a sustained Valsalva manoeuvre. This was repeated at least once in each patient. PFO was defined as a flap-like opening in the atrial septum between the septum secundum and the septum primum allowing permanent or transient right-to-left shunt. ASA was defined as an abnormally redundant septum primum with an excursion of >10 mm into the right or left atrium and a diameter at the base of the aneurysm of at least 15 mm24.
INITIAL PERCUTANEOUS PFO CLOSURE
The initial intervention was performed with mild or no sedation under local anaesthesia and fluoroscopic guidance only, as described previously17. Balloon sizing, intraprocedural guidance by TOE or intracardiac echocardiography was not used in any case. However, most patients had undergone contrast TOE prior to the intervention for initial diagnosis of PFO and anatomical assessment of the interatrial septum. Device selection was at the discretion of the performing physician. Priority was given to the AMPLATZER™ PFO Occluder (Abbott Vascular, Santa Clara, CA, USA, formerly St. Jude Medical) (available since 1997) and the AMPLATZER Cribriform Septal Occluder (Abbott Vascular) due to documented superior performance regarding procedural complications and complete shunt closure16,25.
STUDY ENDPOINTS
Our aim was to evaluate the feasibility and safety of transcatheter residual shunt closure. Feasibility was defined as successful implantation of the device into the correct position at the time the patient left the catheterisation laboratory. Safety was defined as the absence of procedural and in-hospital complications, including vascular injury, bleeding, device embolisation, pericardial effusion, and cerebrovascular events.
FOLLOW-UP EVALUATION
Following successful implantation, transthoracic echocardiography (TTE) was performed before discharge, either on the day of the intervention or the day after, in order to document the correct position of the device. Contrast TOE was recommended after six months to assess closure and exclude thrombi on the device. After a diagnosis of relevant residual shunting, patients were generally scheduled for repeat percutaneous shunt closure which took place 1.4±1.4 months after the diagnosis of residual shunt by TOE. Only exceptionally, a second TOE one to six months later was recommended instead. Until the second intervention the patient was usually left on single antiplatelet therapy, or oral anticoagulation if indicated.
PERCUTANEOUS RESIDUAL SHUNT CLOSURE
The repeat procedure was performed under local anaesthesia and fluoroscopic guidance only. Following an oral dose of a cephalosporin and a bolus of 5,000 U of heparin, a sheathless venous access via the right femoral vein was gained. The residual shunt hole was searched preferably in the left anterior oblique view showing the initial device in profile without overlap of the discs. Angiography of the right atrium or directly of the shunt was performed with the help of a 6 Fr multipurpose catheter in order to localise and further define the defect. If the residual PFO could not be crossed with the catheter, crossing with a regular 0.035” guidewire first and, if unsuccessful, a hydrophilic 0.035” guidewire was attempted. After successful placement of the guidewire in the left atrium, the dedicated delivery sheath (maximum of 8 Fr, according to the occluder selected) was advanced over the guidewire into the left atrium. In case of a second double disc device, the left atrial disc was deployed and pulled back against the atrial septum and the first device under fluoroscopic guidance in a left anterior oblique projection. To deploy the right atrial disc, tension was maintained on the delivery cable while further withdrawing the delivery sheath. Depending on the size and the frequent tunnel configuration of the residual defect, the right device disc often did not resume its disc shape but rather it adopted a cucumber shape (Figure 1).
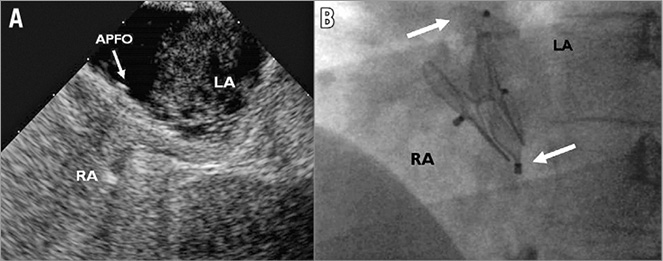
Figure 1. A patient with a relevant residual shunt after implantation of a 35 mm AMPLATZER PFO Occluder (APFO) diagnosed by TOE (A) requiring implantation of a second 25 mm APFO (left anterior oblique fluoroscopic projection, B). The upper arrow shows the left atrial disc, the lower arrow the female screw of the stretched right atrial disc of the second device having adopted a cucumber shape. LA: left atrium; RA: right atrium; TOE: transoesophageal echocardiography
In selected cases with a residual tunnel shunt between the discs of the first device, an AMPLATZER™ Vascular Plug (Abbott Vascular) was deployed in the tunnel. For this, a diagnostic catheter sufficed and passage into the left atrium was not required (Figure 2).
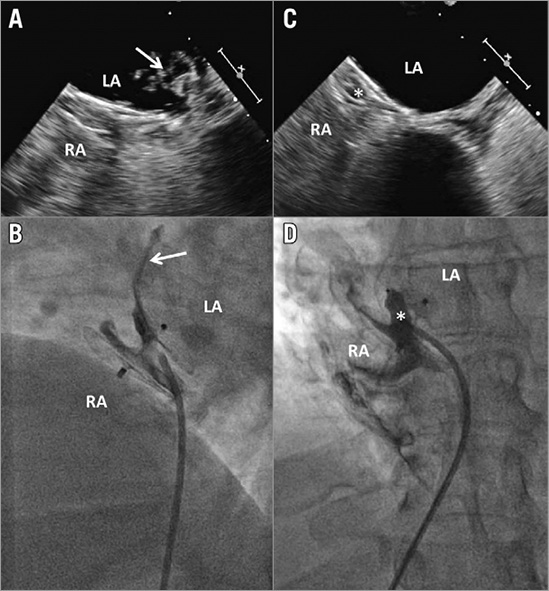
Figure 2. Patient with residual shunt grade 2 treated by implantation of an AMPLATZER Vascular Plug 8 mm (*). A) & B) Relevant residual shunt documented by follow-up transoesophageal echocardiography (TOE) (A) and fluoroscopy (B)(arrows). C) & D) Successful closure confirmed immediately by fluoroscopy (D) and at six months by TOE (C; right atrium [RA] filled with agitated saline and contrast medium). LA: left atrium
Right atrial contrast angiography through the delivery sheath or catheter was used for control of the correct position of the second device by delineating the atrial septum in a projection depicting the devices in profile, avoiding overlay of the two discs of each individual device. Finally, the catheter was removed and haemostasis achieved by manual compression, typically performed by the patient. As after the first intervention, patients were released to full physical activity as early as a few hours after the procedure. TTE was performed before discharge in order to document the correct and stable position of the devices. A second dose of a cephalosporin was given a few hours after the procedure and, in case of an overnight hospitalisation, a third one the following morning. Acetylsalicylic acid 100 mg/d for five to six months and clopidogrel 75 mg/d and endocarditis prophylaxis for one to three months were prescribed.
STATISTICAL ANALYSIS
The statistical analysis considered all patients scheduled for implantation of a second PFO occluder during the study period, including the patients in whom the defect could not be crossed. Continuous variables are expressed as mean±standard deviation, and were compared by a two-sided, unpaired t-test. Categorical variables are reported as counts and percentages, and were compared using Fisher’s exact test. Binary logistic regression analysis was performed to identify independent predictors of the need for a second device. Estimates of the odds ratio (OR) and 95% confidence intervals (CI) for each independent variable were obtained by proportional hazard regression analysis. Statistical significance was assumed with a p-value <0.05. All data were analysed with the use of SPSS software, Version 21 (IBM Corp., Armonk, NY, USA).
Results
INITIAL PROCEDURE
The indication for initial PFO closure in the 100 patients referred for residual shunt closure was ischaemic stroke in 54 patients, TIA in 31, peripheral embolism in four, diving accident in three, migraine in one, and other in seven (Table 1). Ten different devices were used for initial PFO closure (AMPLATZER PFO Occluder in 54 patients, AMPLATZER Cribriform Occluder in 28, PFO-Star Occluder in seven [Cardia Inc., Eagan, MN, USA], Occlutech® Figulla® Occluder in four [Occlutech GmbH, Jena, Germany], Sideris Buttoned Occluder in two [Custom Medical Devices, Amarillo, TX, USA], as well as AMPLATZER™ ASD Occluder [Abbott Vascular], Premere Occluder [St. Jude Medical, St. Paul, MN, USA], Nit-Occlud® [B. Braun, Melsungen, Germany], Angel Wings™ Occluder [Microvena Corporation, White Bear Lake, MN, USA], and Coherex FlatStent® [Coherex Medical, Salt Lake City, UT, USA] in one patient each). The proportion of patients requiring transcatheter residual shunt closure was strongly dependent on the type of device used during the first intervention, ranging from 3.2% for the AMPLATZER PFO Occluder to 11.5% for the Cardia PFO-Star Occluder (Figure 3). Compared to the whole population (N=2,679), a significantly higher rate of ASA (58% versus 36%; p=0.024), a larger proportion of shunt grade 3 at baseline (97% versus 78%; p<0.001), and a larger size (≥30 mm) of the first implanted device (47% versus 13%; p<0.001) were observed in the patients with relevant residual shunt. In addition, the use of a larger device size was found to be an independent predictor of the need for a second device (OR 4.1, 95% CI: 1.6-10.0; p=0.002) (Table 2). Six patients (6%) experienced a recurrent cerebrovascular event before the second intervention (three ischaemic strokes and three TIAs). The earliest cerebrovascular event occurred six months and the latest more than seven years after the first procedure. Four patients had a 30 mm AMPLATZER PFO Occluder, one patient a 35 mm AMPLATZER PFO Occluder, and one patient a 35 mm AMPLATZER Cribriform Occluder in place. Concerning residual shunt severity, four patients had shunt grade 3, and two shunt grade 2. When compared to recurrent cerebral events in patients without residual shunt, statistical analysis showed a trend towards significance in favour of the patients without residual shunt (4% versus 6%; p=0.074). Accounting for the fact that the mean follow-up time was twice as long in the group without residual shunt (4.3 years versus 2.1 years), this represents an about three times higher annual rate for the patients with residual shunt. At the time of recurrence, none of the occluders showed thrombus at TOE and repeated standardised work-up did not detect alternative causes for the recurrent events. All patients with recurrent events had continuous antithrombotic therapy until residual shunt closure occurred (three patients had acetylsalicylic acid, one clopidogrel, one both, and one rivaroxaban).
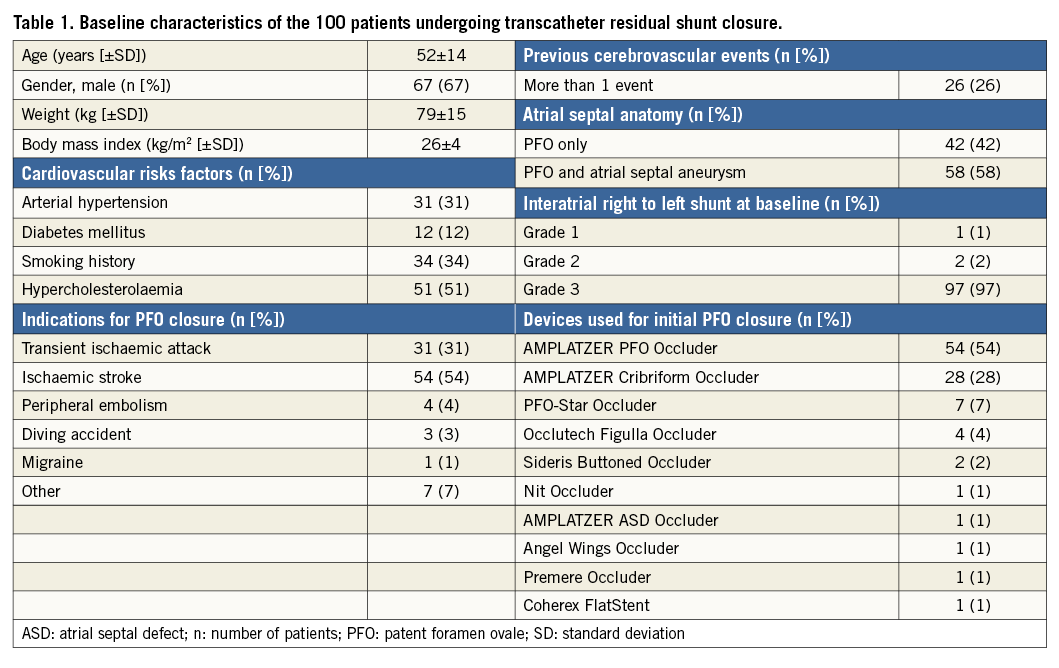
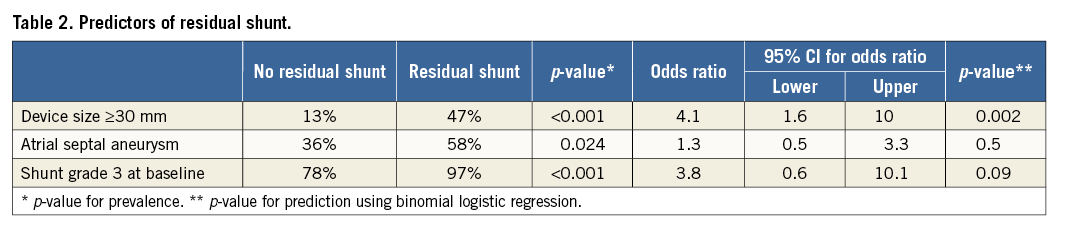
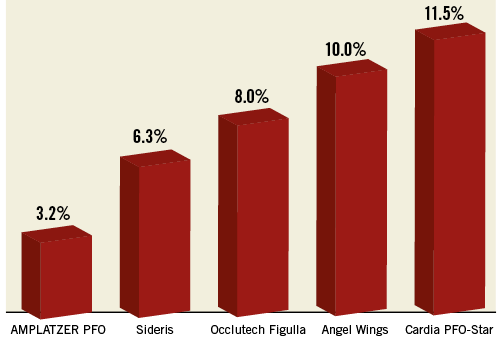
Figure 3. Rate of reintervention according to device type.
TRANSCATHETER RESIDUAL SHUNT CLOSURE
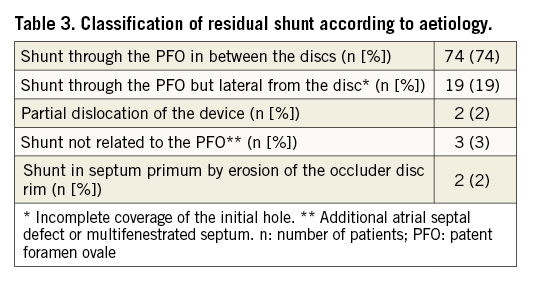
The severity of residual shunt in the 100 patients referred for residual shunt closure is shown in Figure 4A. The second intervention took place 14±13 months after initial percutaneous PFO closure. In 26 patients, the intervention was performed more than one year after the initial procedure. A shunt in between the discs of the first device was found in 74 patients and a shunt lateral from it (due to incomplete coverage of the PFO width) was observed in 19 patients (Table 3). Follow-up TOE showed a hitherto missed additional ASD in an ASA as the cause of the residual shunt in one patient, and in two additional patients a multifenestrated (“Swiss cheese”) septum primum was diagnosed. Dislocation of the cranial part of the right-sided disc in the PFO tunnel was identified in two patients as the cause of residual shunt. Finally, two patients had a new iatrogenic ASD caused by erosion of the septum primum at the caudal rim of the disc26,27.
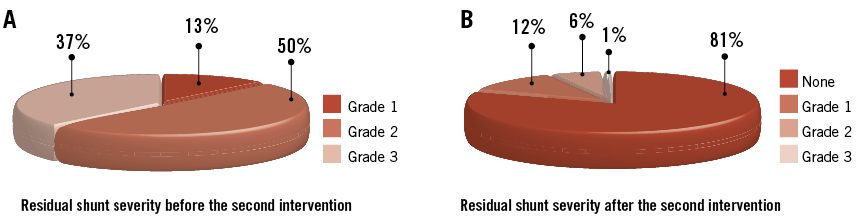
Figure 4. Residual shunt severity. Before (A) and late after (B) transcatheter residual shunt closure.
Residual shunt closure failed in two patients due to inability to find (one patient) or cross (one patient) the defect. Among the remaining 98 patients, nine different devices were used for the second intervention. An AMPLATZER PFO Occluder was successfully implanted in 74 patients (76%), an AMPLATZER Vascular Plug in 10 (10%), an AMPLATZER Cribriform Occluder in four (4%), an AMPLATZER ASD Occluder in three (3%), a Sideris Occluder in two (2%), a Cera™ Occluder in two (2%) (Lifetech Scientific [Shenzhen] Co., Ltd., Shenzhen, China), and a Figulla Occluder, a CardioSEAL® Occluder (NMT Medical, Boston, MA, USA), and a Hyperion™ Occluder (Comed B.V., Heerenveen, the Netherlands) in one patient (1%) each. Decision on device type (second occluder or AMPLATZER Vascular Plug) was made according to the fluoroscopic appearance of the defect. Mean intervention duration was 26±20 minutes and mean fluoroscopy time 9±10 minutes. The mean amount of contrast medium used was 84±60 ml. These values did not significantly differ from those recorded for the baseline interventions. There were no procedural or late complications.
ECHOCARDIOGRAPHIC OUTCOME
In the 98 patients with successful residual shunt closure, TOE was obtained in 88 patients (90%) after 7±5 months. Among them, complete closure was achieved in 81%. Minimal residual shunt persisted in 12%, grade 2 in 6%, and grade 3 in 1% (Figure 4B). In eight patients (two patients with residual shunt grade 1 [patient wish], five with grade 2, and one with grade 3), corresponding to 0.3% of the whole cohort (N=2,679), a third procedure was performed achieving complete closure at follow-up TOE in all (Figure 5).
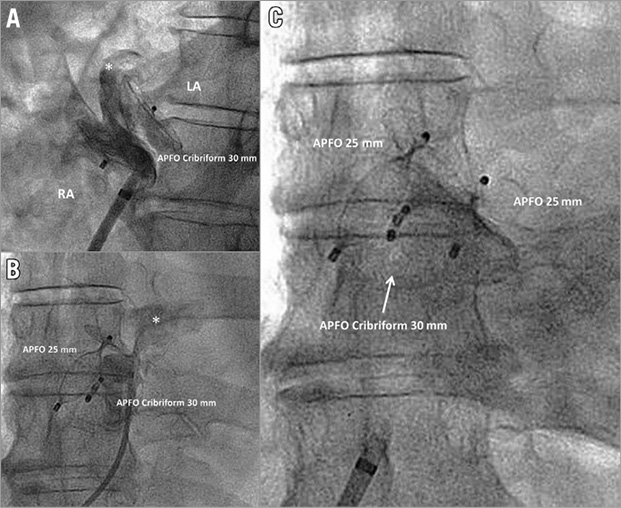
Figure 5. Patient with three AMPLATZER PFO Occluder (APFO) devices implanted during three separate procedures. A) Residual shunt (*) late after initial implantation of a 30 mm APFO Cribriform. B) Relevant residual shunt (*) late after implantation of a second 25 mm APFO. C) Implantation of a third 25 mm APFO for closure of a remaining shunt in the direct inflow line of the inferior vena cava, resulting in complete closure at six months. LA: left atrium; RA: right atrium
Discussion
Transcatheter closure of a residual shunt after percutaneous PFO closure is rarely performed and data to guide clinical decisions are sparse18-23. However, an association between the presence of a residual shunt and the risk of recurrent events has been established. Moreover, a randomised trial investigating the long-term clinical outcomes with three different closure devices (AMPLATZER, CardioSEAL/STARFlex, and Helex® [W.L. Gore & Associates, Inc., Flagstaff, AZ, USA] occluders) found a significantly higher recurrence rate in the patients treated with CardioSEAL/STARFlex and Helex occluders, associated with an increased incidence of residual shunt25. As a consequence, moderate to severe residual shunt should be considered a relevant clinical issue requiring therapeutic attention.
In this study, patients with residual shunt showed a more than twofold increased annual risk of recurrent cerebrovascular events when compared to a similar cohort of 620 patients followed during one year (2.9% versus 1.3%)28. In addition, there was a trend towards significance concerning the cerebral events rate favouring the patients without residual shunt. This effect became more pronounced (almost threefold) when adjusting for the length of follow-up. However, it has to be mentioned that this may, at least partly, be explained by a selection bias resulting from the fact that patients with recurrent events in the presence of residual shunt were more likely to be referred for transcatheter residual shunt closure and to consent to a second intervention. The time delay of recurrent stroke (six months to seven years) observed in this cohort rather speaks against a cause related to device or procedure.
From a mechanistic point of view, there are three distinct mechanisms potentially favouring the persistence of a residual shunt. First, larger devices will not hug the septum snugly and there might be a remaining tunnel between the device discs. This mechanism is supported by our data which identified the use of devices ≥30 mm as a strong predictor of the need for a second device (OR 4.1, 95% CI: 1.6-10.0; p=0.002). Second, the selected occluder may be too small, with a right disc part dislocating into the PFO channel or just not covering the entire width of the PFO slit with a residual shunt adjacent to the device. Third, an erosion of the septum primum at the device rim may exceptionally result in an iatrogenic atrial septal defect appearing as a residual shunt26,27. The risk for all of these mechanisms of residual shunt may be aggravated by the presence of an ASA which has been repeatedly associated with the occurrence of a residual shunt in the literature28-31. In line with these findings, the majority of the patients requiring a second device had an ASA in our study (58%). In one study, large shunt size has been identified as an additional predictor of incomplete closure29.
From a technical point of view, important discrepancies between the performances of the different devices have been reported16,25 and are confirmed by our study (Figure 3). Thus, appropriate device selection is crucial to minimise the risk of residual shunt.
Of note, in rare cases, residual shunting may be related not to the PFO itself but to a hitherto missed additional ASD, a multifenestrated septum, or a pulmonary arteriovenous malformation. In our 100 patients, only five were found to have additional defects, other than the PFO, causing a residual shunt. Pulmonary arteriovenous malformations are typically associated with delayed right-to-left shunt during TOE coming from the region of the pulmonary veins and not directly from the septum. Thus, particular attention should be paid to document the passage of the echocardiographic contrast medium through the septum itself after a Valsalva manoeuvre in order to exclude this pathology. In our series, no patient was found to have pulmonary arteriovenous malformation.
Beyond the medical considerations, the psychological dimension also needs to be considered. Indeed, the patient assumes that, if the interatrial communication, probably causative of the initial event, persists, this represents a continuing menace. As shown in our series, complications of the second procedure are not to be expected and the chances of this being the last one required are excellent, considering that only 0.3% of the patients underwent a third procedure in our large cohort.
This consecutive series of patients with a second catheter-based PFO occlusion is the largest one reported so far. It documents the safety and high success rate of repeat PFO closure, particularly when using AMPLATZER devices. The clinical outcome is rewarding but in the absence of a control group a clinical benefit of second closure cannot be derived.
Limitations
Several limitations need to be considered when interpreting the results of this study. First, the study population reflects a uniform technique, albeit with different devices, with fluoroscopic guidance only. It may not be easily comparable to other closure techniques. As some series have reported late spontaneous residual shunt closure, the incidence of residual shunt might have been slightly lower if the follow-up TOE had been scheduled later. The inter-device comparisons shown in Figure 3 need to be interpreted with caution since the different devices have been used with different frequencies and at different points in time of our learning curve (mainly explained by their chronological availability for commercial use). Finally, the increased incidence of recurrent cerebral events observed in this cohort may be at least partly related to selection bias, resulting from the fact that patients with recurrent events in the presence of residual shunt were more likely to be referred for transcatheter residual shunt closure and to consent to a second intervention.
Conclusions
Transcatheter residual shunt closure after initial percutaneous PFO closure can be safely performed under fluoroscopic guidance only. It achieves complete closure in most patients. A very small proportion of patients requires a third intervention. The use of larger devices, typically prompted by intricate anatomy, represents a risk factor for shunt persistence and the need for reintervention.
| Impact on daily practice The management of residual shunt following percutaneous PFO closure has been poorly investigated so far. Transcatheter residual shunt closure after initial percutaneous PFO closure is safe and results in complete closure in most patients. Large devices should be used in complex anatomical situations only, as they are associated with an increased risk of residual shunt. |
Conflict of interest statement
S. Windecker has received research grants to his institution (Bern University Hospital) and from Abbott, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic, and The Medicines Company. F. Nietlispach is a consultant and proctor for Abbott. B. Meier has received speaker and proctor honoraria from Abbott. The other authors have no conflicts of interest to declare.

