
Abstract
Aims: Although three recent trials have shown a significant stroke risk reduction after tPFOc, the individual statistical power is limited and the impact on pooled evidence needs to be explored. We aimed to pool data from available randomised clinical trials (RCT) to assess whether tPFOc is more effective and safe than antithrombotic therapy alone (ATA).
Methods and results: Major electronic databases and tangential sources were searched. Six trials (3,560 patients) were identified. At a median follow-up of 3.6 (2.0-5.2) years (13,930 person-years), the risk of stroke was significantly lower after tPFOc compared with ATA (HR 0.28, 95% CI: 0.12-0.64, p=0.003). Significant heterogeneity was detected (I2=66.1%), although single trials did not significantly influence the results. Reconstructed time-to-event data revealed that tPFOc benefits accrue after approximately one year and persist over time without significant variations (96.4% versus 88.0%; HR 0.25, 95% CI: 0.09-0.66, p=0.005; NNT=11). Although results showed a greater benefit in patients <45 years old, male, and with substantial shunt, interaction between subgroups was not significant. Trial sequential analysis showed that accumulated evidence appeared to be sufficient. However, tPFOc did not confer protection against transient ischaemic attack (TIA; HR 0.69, 95% CI: 0.31-1.54, p=0.365) and a significant excess in the risk of atrial fibrillation was observed (OR 4.99, 95% CI: 1.99-10.10, p<0.001), though generally early and transient. Major bleeding and migraine were comparable between treatments.
Conclusions: Compared with ATA, tPFOc significantly reduces the risk of stroke at long-term follow-up but no benefit is observed in terms of TIA. Atrial fibrillation is higher after tPFOc, though generally early and transient. The risks of major bleeding and migraine are comparable between the groups.
Abbreviations
ATA: antithrombotic therapy alone
CI: confidence interval
HR: hazard ratio
NNT: number needed to treat
OR: odds ratio
PFO: patent foramen ovale
RCT: randomised clinical trials
tPFOc: transcatheter patent foramen ovale closure
TIA: transient ischaemic attack
Introduction
Patent foramen ovale (PFO) is associated with an increased risk of stroke as a result of paradoxical cerebral embolism1,2. Transcatheter PFO closure (tPFOc) is an attractive preventive approach but its effectiveness compared with antithrombotic therapy alone (ATA) has remained a matter of debate for a long time3,4. Indeed, early randomised clinical trials (RCT) did not prove a significant reduction in stroke compared with ATA4-7, although later a pooled analysis and observational data fostered a possible benefit of tPFOc4,8,9. Currently, the European Stroke Organisation indicates tPFOc only in patients with cryptogenic stroke and PFO with high-risk features, while the American Heart Association/American Stroke Association guidelines consider tPFOc a viable alternative to ATA only in patients with PFO and recurrent deep vein thrombosis10,11.
Recently, the results of the CLOSE, Gore REDUCE, and DEFENSE-PFO trials12-14 showed significant risk reductions in stroke after tPFOc. Because all of the existing trials have low statistical power to detect difference in stroke rates and questions on secondary efficacy and safety endpoints remain unanswered, an updated systematic review represents a relevant undertaking. The several meta-analyses on the topic published over time present (with mixed proportions) the following limitations: absence of one or more of the available RCT, biased selection of reports, evidence based mostly on observational studies, inappropriate definition of the risk by estimates not accounting for time, missing or incomplete assessment of the risk variation over time, insufficient exploration of the heterogeneity across trials and clinical subgroups, omitted quantification of statistical power of the accumulated evidence, and incomplete definition of the net benefit of tPFOc5,15-19.
Against this background, we aimed to provide an updated meta-analysis on tPFOc versus ATA to quantify the impact of the three newer trials, and to provide a proper description of risk variation in relation to time, and a critical assessment of the net benefit of tPFOc.
Methods
We conducted a frequentist pairwise meta-analysis in keeping with the recommendations of PRISMA (Supplementary Table 1) and the Cochrane Collaboration20,21. This meta-analysis was registered with PROSPERO (www.crd.york.ac.uk/prospero/; CRD42017081518).
ELIGIBILITY CRITERIA AND LITERATURE SEARCH
Three authors (N. Caronna, A.H. Frangieh, J. Michel) independently searched PubMed, Scopus, Web of Knowledge, ScienceDirect and Ovid electronic databases from the inception to 1 December 2017. No language restrictions or specific clinical subsets were imposed. A complementary search was performed by accessing major scientific websites with interest in the topic (www.clinicaltrials.gov, www.clinicaltrialresults.org, www.tctmd.com, www.pcronline.com, www.acc.org, www.heart.org) and screening of bibliographies of relevant reviews and book chapters. Duplicates due to the multiple-database search were removed. The retrieved data set was used for preliminary assessment of the feasibility of the meta-analysis and for qualitative definition of each of the included trials (Supplementary Appendix 1).
According to PRISMA recommendations20, after data extraction, performance of the statistical analysis, and manuscript drafting, a last search was made on 15 March 2018. The identification of an additional trial that met eligibility criteria required an update of the meta-analysis.
ENDPOINTS
The primary endpoint was stroke at the longest available follow-up. The secondary endpoints included transient ischaemic attack (TIA), atrial fibrillation, major bleeding and migraine at the longest available follow-up.
DATA EXTRACTION
Authors involved in the search (N. Caronna, A.H. Frangieh, J. Michel, D. Giacoppo) extracted trial-level qualitative and quantitative data. Trial-level risk estimates, incidences of events, and numbers of patients were exported for statistical analysis. Intention-to-treat analyses were considered. All the authors had full access to the data.
STATISTICAL ANALYSIS
Categorical variables are presented as proportions (counts) and were tested using the χ2 test. Continuous variables are presented as means (standard deviations) and were compared using the t-test or ANOVA. Within-trial and between-trial means and standard deviations were weighted.
According to original long-term time-to-event analyses, trial-level hazard ratios (HRs) and 95% confidence intervals (CIs) were used for stroke and TIA. Pooled risk estimates were computed by fixed-effect and random-effects models with inverse variance weighting21,22. Risk distribution across trials was illustrated by forest plots with weighting according to a random-effects model21,22. The number needed to treat (NNT) was estimated as previously described for survival analysis22,23.
We assessed heterogeneity by using Cochran’s Q test with significance set at 0.10, between-study variance τ², and the I² statistic21,22,24. I² values <25% expressed low heterogeneity, 25-50% moderate heterogeneity, and >50% high heterogeneity24.
We reconstructed time-to-event data for the primary endpoint of stroke by extreme-magnification digitisation of the original high-quality Kaplan-Meier curves and then by modelling of retrieved spatial information along with numbers of events and numbers at risk for each time interval25,26. Additional information is provided in Supplementary Appendix 2. Retrieved data were used to carry out Kaplan-Meier analyses. According to a “one-stage” meta-analysis, a mixed-effect Cox proportional hazard regression model taking into account the original clustering of patients across trials was used to provide risk estimates alternative to those obtained by standard aggregate-data meta-analysis27. A “two-stage” meta-analysis with trial-level estimates according to Cox regression was also performed. Differences between treatments were compared by log-rank test.
A restricted maximum likelihood random-effects multiple-outcome meta-analysis was performed to provide a joint estimate of the risk of ischaemic stroke and TIA28. We decided to apply such a type of inference because it can provide estimates taking into account the correlation between outcomes and overcome missing values (“borrowing of strength”)28.
Risks of atrial fibrillation, major bleeding and migraine between groups were expressed by odds ratios (ORs) and 95% CIs, since these outcomes were not uniformly reported according to time-to-event analyses across trials and most of them occurred early.
Analyses were performed using R 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) and Stata 13 (StataCorp, College Station, TX, USA).
SENSITIVITY AND SUBGROUP ANALYSES
The influence of individual trials on pooled estimates and heterogeneity was explored by removing each one at a time (“leave-one-out”)29.
Time-to-event landmark analyses were performed to compare the distributions of the events between groups from enrolment to two-year follow-up and from two years after enrolment to maximum available follow-up. In addition, after excluding trials having shorter follow-up, we assessed the risk of stroke between groups at five years to remove possible influences of trials designed to assess outcomes at earlier time points and mitigate the impact of very late follow-up variations across trials.
A cumulative meta-analysis was conducted to inspect the variations after addition of each trial in a chronological order and an O’Brien-Fleming monitoring boundary by using the Lan-DeMets alpha spending function approach (“trial sequential analysis”) was computed to exclude spurious effects30. In addition, pooled effects of earlier and newer trials were compared to quantify the contrast between early and recent results.
Consistency of main results was assessed across the subgroups of age <45 versus ≥45 years, male versus female, and substantial versus non-substantial shunt according to large or mild-to-moderate transit of microbubbles, respectively. Interaction between effects was assessed with significance set at 0.05.
Finally, the risk of TIA was also assessed by using OR according to the reported number of events.
BIAS ASSESSMENT
Trial-level qualitative assessment was performed by using the Cochrane Collaboration tool21, and the robustness of the meta-analysis conclusions was defined according to GRADE31. Further specifications on the two tools are reported in Supplementary Appendix 2.
Results
The search process is illustrated in Supplementary Figure 1 and details of the strategy applied are shown in Supplementary Table 2. Six RCT5-7,12,13,32 including 3,560 patients (1,889 tPFOc versus 1,671 ATA) were included in the meta-analysis. The main characteristics are reported in Table 1, while eligibility criteria are listed in Supplementary Table 3. Baseline characteristics were balanced between groups (Supplementary Table 4), though there were differences across trials (Supplementary Table 5). Overall, the included patients were middle-aged adults (45.3±9.9 years), with similar distribution between genders and low cardiovascular risk profile. Almost all patients had a recent cryptogenic stroke as qualifying event and frequently had large shunting. Devices and ATA regimens across trials are reported in Table 1 and Supplementary Table 6.
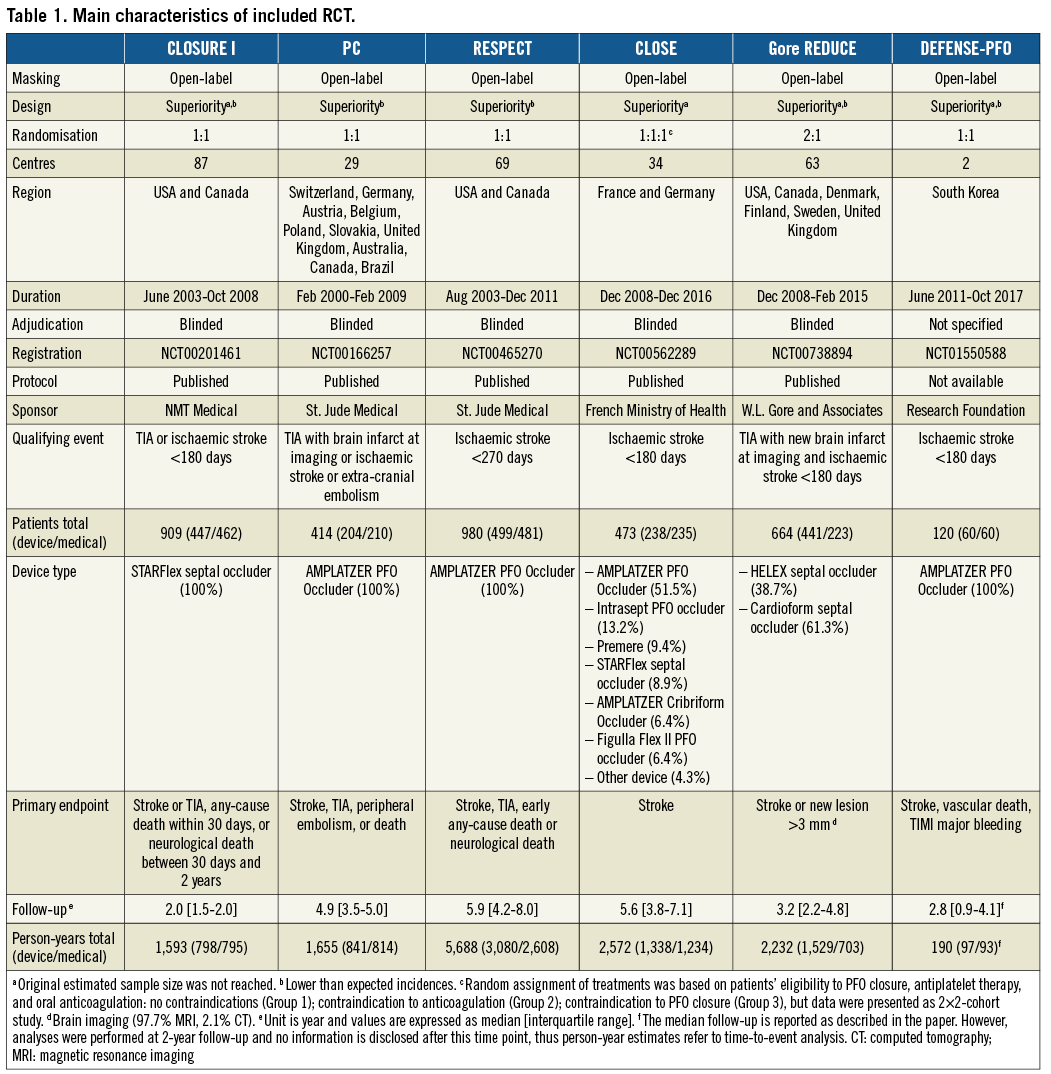
STROKE
At a median follow-up time of 3.6 [2.0-5.2] years, 116 events occurred, 37 after tPFOc and 79 after ATA. Regardless of the model applied, the risk of stroke was significantly lower after tPFOc compared with ATA (HR 0.28, 95% CI: 0.12-0.64, p=0.003) (Figure 1). The relative weight of trials was balanced overall. However, a high degree of heterogeneity was observed (I2=66.1%), reflecting the different magnitude of trial-level effects and 95% CIs rather than direction. Indeed, all point estimates were to the left of the null: the CLOSURE I trial5 showed no difference between strategies, the PC and RESPECT trials6,7,32 showed a numerical benefit of the interventional treatment, while the CLOSE and Gore REDUCE trials12,13 showed a significant risk reduction after tPFOc. Regardless of the model applied, no single trial could significantly influence pooled estimates (Figure 2) and between-trial heterogeneity remained high in any case.
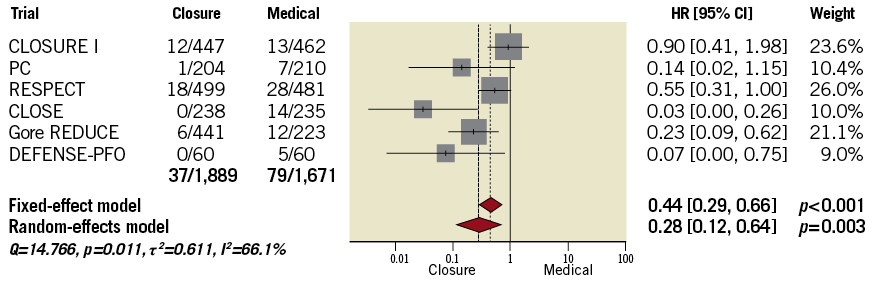
Figure 1. Comparison between tPFOc and ATA at the longest available follow-up.
Figure 2. Influence analysis.
Kaplan-Meier curves showed that difference between treatments emerged after approximately one year (Figure 3). The survival free from stroke at the maximum available follow-up (13,930 person-years) was 96.4% after tPFOc (7,683 person-years) and 88.0% after ATA (6,247 person-years). The annualised incidence of stroke was 0.48/100 person-years after tPFOc and 1.26/100 person-years after ATA. The meta-analysis of reconstructed time-to-event data provided results consistent with aggregate-data meta-analysis (HR 0.25, 95% CI: 0.09-0.66, p=0.005). It was estimated that approximately 11 patients needed to undergo PFO closure to prevent one stroke as compared with ATA (NNT 11.3, 95% CI: 9.2-25.6). The landmark analysis showed uniform distributions over time, and risk estimates by “one-stage” meta-analyses were consistent with “two-stage” meta-analyses (Figure 4).
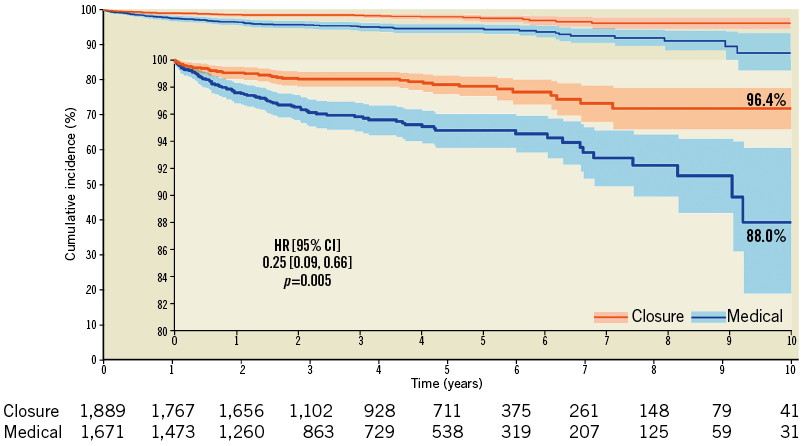
Figure 3. Survival free from stroke.
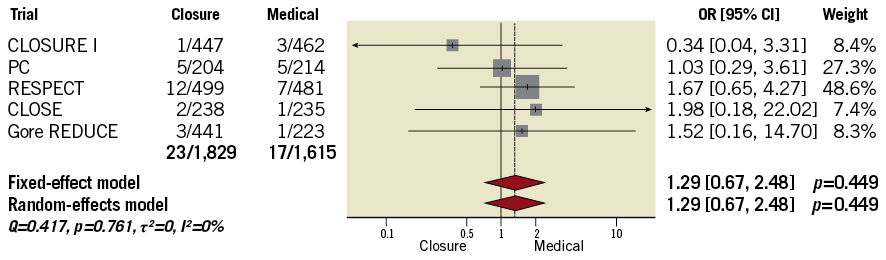
Figure 4. Landmark analysis. The risk estimates within Kaplan-Meier graphs are derived from mixed-effects Cox proportional hazards regression accounting for the original clustering of patients (“one-stage”). The forest plots illustrate sensitivity analyses according to standard Cox proportional hazards regression and subsequent combination of trial-level outcomes by fixed-effect or random-effects models (“two-stage”).
The definition of stroke showed acceptable consistency across trials (Supplementary Table 7). Events, events/100 person-years, and Kaplan-Meier estimates across trials and time points are reported in Supplementary Table 8.
With the aim of excluding investigations implying outdated and potentially ineffective devices and including only trials achieving very long-term data, we excluded the CLOSURE I and DEFENSE-PFO trials5,14. At five-year follow-up, no significant changes in the main conclusion were noted regardless of the method applied (“one-stage”, mixed-effects Cox regression: HR 0.22, 95% CI: 0.09-0.54, p=0.001; “two-stage” random-effects: HR 0.23, 95% CI: 0.09-0.58, p=0.002) (Figure 5). Results by including all trials were consistent (Supplementary Table 8).
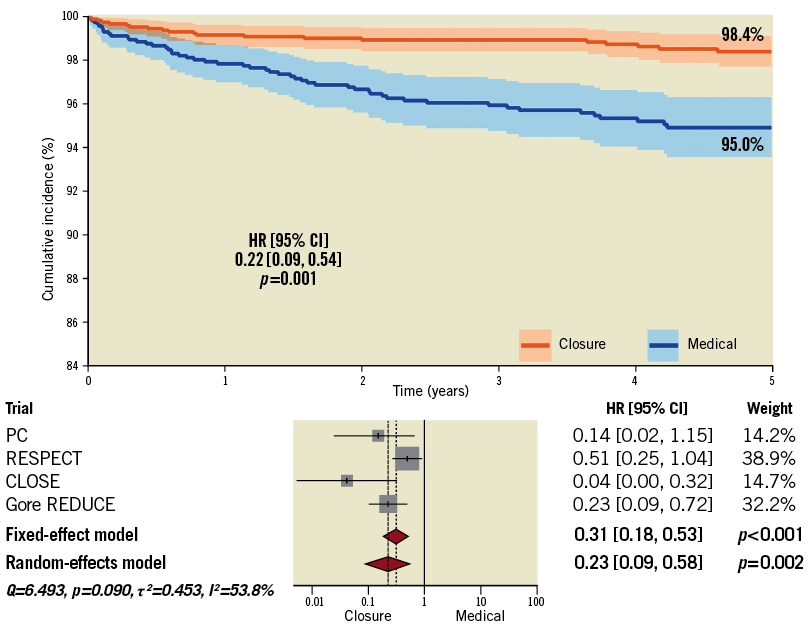
Figure 5. Five-year analysis after exclusion of trials with outdated devices and limited follow-up.
Trial sequential analysis was performed to exclude spurious results due to type I error and to assess the statistical power of pooled data (Figure 6). While a conventional significance threshold (z=1.96; solid green line) was not reached by pooling the three earlier trials5-7, at cumulative analysis the addition of the CLOSE trial12 produced a significant variation (p=0.043) that would be spurious when accounting for type I error. After the addition of the Gore REDUCE trial13, the cumulative z curve (z=2.599) crossed the alpha spending function monitoring boundary (z=2.075; dashed red line). The relative weight of the Gore REDUCE trial13 on cumulative effect was greater than that of the CLOSE trial12 (Supplementary Figure 2) and, after switching the order of addition of the two trials, the cumulative z curve (z=2.284) crossed the monitoring boundary earlier (z=2.243).
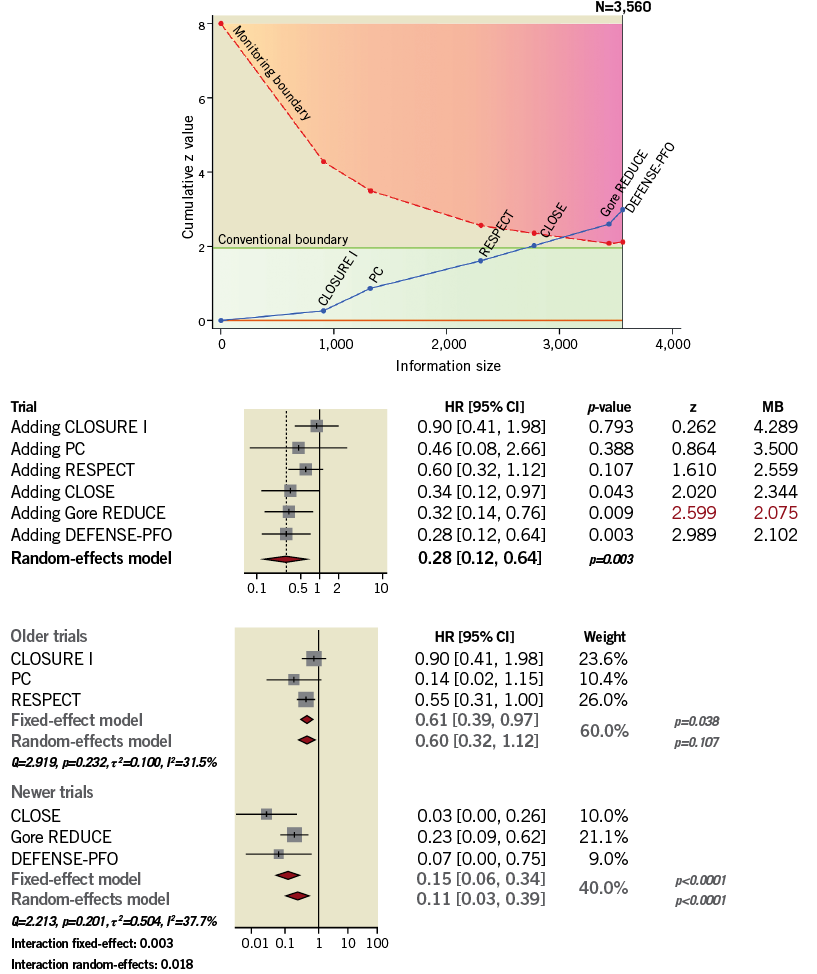
Figure 6. Trial sequential analysis, cumulative meta-analysis and comparison between earlier and newer trials. Red numbers indicate when the cumulative risk reaches statistical significance. MB: monitoring boundary
At subgroup analysis (Figure 7), the stroke risk reduction after tPFOc seemed to be larger in patients <45 years old, males, with substantial shunt. However, no interaction between subgroups was observed.
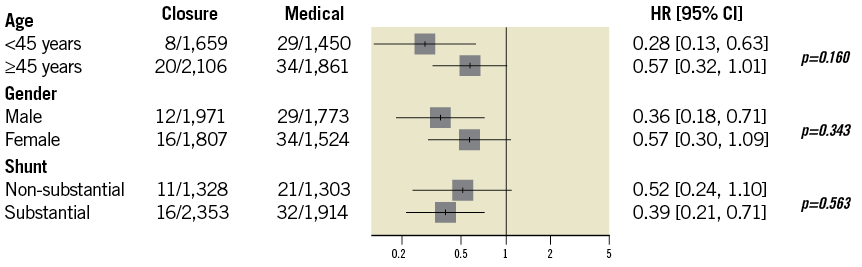
Figure 7. Subgroup analyses.
TIA
The risk of TIA was not significantly reduced after tPFOc compared with ATA (HR 0.69, 95% CI: 0.31-1.54, p=0.365) (Figure 8). The univariate sensitivity analysis using the number of events (ORs) was consistent (Supplementary Figure 3). No heterogeneity across trial-level estimates was observed (I2=0%; p=0.984).
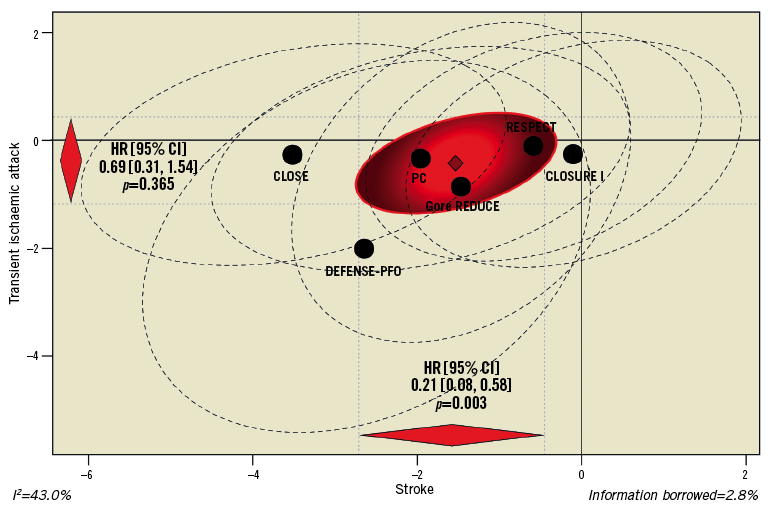
Figure 8. Multiple-outcome meta-analysis of stroke and TIA.
ATRIAL FIBRILLATION AND MAJOR BLEEDING
Compared with ATA, tPFOc was associated with a more than fourfold increase in the risk of atrial fibrillation (Figure 9A). Heterogeneity was moderate (I2=44.8%; p=0.124). The risk of major bleeding was overall low and comparable between treatments (Figure 9B). Heterogeneity was not significant (I2=34.5%; p=0.191). Rates of other major cardiovascular adverse events are summarised in Supplementary Table 9.
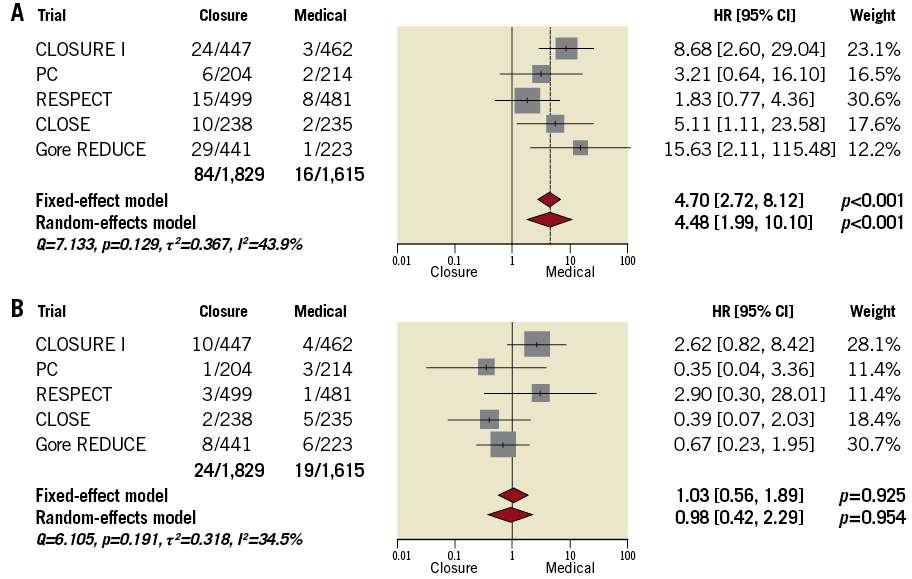
Figure 9. Atrial fibrillation and major bleeding. A) Atrial fibrillation. B) Major bleeding.
MIGRAINE
Compared with ATA, tPFOc did not seem to produce any benefit in terms of migraine (Supplementary Figure 4).
BIAS ASSESSMENT AND STUDY RELIABILITY
Overall, the quality of the included trials was moderate-to-high, but some possible sources of bias need to be taken into account, as illustrated in Supplementary Figure 5. According to GRADE31, the reliability of our conclusions is acceptable (Supplementary Table 10).
Discussion
The findings of this meta-analysis can be summarised as follows: 1) compared with ATA, tPFOc reduces the risk of stroke at very long-term follow-up; 2) the results are robust, do not depend on individual trials and do not change across analyses accounting for multiple testing and clinical subgroups; 3) although substantial heterogeneity was observed, this depended on differences in magnitude rather than direction of treatment effects; 4) although pathophysiologically correlated with stroke, tPFOc does not protect from TIA; 5) tPFOc imposes a higher post-procedural risk of atrial fibrillation, while no difference in major bleeding was observed; 6) no benefit of tPFOc against migraine is observed.
A major finding of our study was that the relative risk reduction of stroke was detectable after approximately one year after enrolment and continued to accrue with increasing duration of surveillance. Considering that each trial has no power for stroke (i.e., design for composite endpoints, anticipated termination, lower than expected incidence of events), extended follow-up enables capturing a larger number of events. The non-significant results observed in the primary analyses of the PC and RESPECT trials6,7,32 should be interpreted against this background; over a short-to-medium time horizon, the incidence of stroke was insufficient to prove the benefit of tPFOc, as suggested by secondary analyses with extended follow-up4,32.
However, this finding can only partially explain the different results observed between earlier and newer trials. Indeed, several factors might have contributed to the strong conclusions of the recent CLOSE, Gore REDUCE, and DEFENSE-PFO trials12-14, despite sample size or follow-up time comparable with previous trials. First, the selection of patients in newer trials may have played a key role. In the CLOSURE I and PC trials5,6, a recent TIA or peripheral embolism could justify enrolment, while in the other trials7,12-14,32 almost exclusively patients who experienced cryptogenic stroke were considered for inclusion. Moreover, in earlier trials5-7, a relevant proportion of patients - about 50% in the CLOSURE I trial5 - had a small shunt, while in recent trials12-14 patients with a substantial shunt were mostly enrolled. Second, in the CLOSE and in other recent trials12-14, the longer experience gained may have led to more effective procedures by proper anatomic assessment and device size selection. Third, differences in devices and medical therapy may have influenced the results of trials. The CLOSURE I trial5 employed the use of a double umbrella-like occluder comprised of a nickel-cobalt framework with attached polyester fabric that might not have worked as well as other devices5,33,34. Consistent with observational data, the three trials7,14,32 based on a double-disc occluder device comprising a self-expanding nitinol mesh with a sewn polyester patch showed not only improved results but also fewer complications. Similarly, the Gore REDUCE trial13, which tested double-disc occluders comprising a platinum-filled nitinol wire frame covered with expanded polytetrafluoroethylene, showed a strong reduction in the risk of stroke after tPFOc. However, in the CLOSE trial12 –the investigation showing the most pronounced stroke risk reduction and the absence of stroke in the tPFOc group– several available devices were implanted in mixed proportions.
The impact of different ATA regimens in patients allocated to conservative treatment should also be considered. In the CLOSURE I, PC, and RESPECT trials5-7, as well as in observational studies35, outcomes were comparable between patients receiving anticoagulation and antiplatelet therapy. In the CLOSE trial12, the randomised comparison of anticoagulation versus antiplatelet therapy showed a numerical trend favouring anticoagulation. Conversely, in the RESPECT trial7,32, there was a significant risk reduction associated with antiplatelet therapy, although interaction testing was borderline. No information was provided for the DEFENSE-PFO trial14.
We also performed landmark time-to-event analysis to provide estimates accounting for the overall significant loss of patients at follow-up and to assess consistency of results over time. Indeed, a significant proportion of patients were right-censored from two to five years, probably as a result of the limited per-protocol follow-up requirements of the CLOSURE I, Gore REDUCE and DEFENSE-PFO trials5,13,14 and originally unplanned very late follow-up in the RESPECT trial32. In addition, the proportion of patients who were lost at follow-up was higher in the ATA group4. The significant proportion of patients with incomplete follow-up represents an important limitation of trials and must be borne in mind when interpreting very late outcomes. Nevertheless, our analysis showed evidence that the curves continue to diverge after two years and the number of events in the ATA group increases over time.
Importantly, some authors might argue about the inclusion of the CLOSURE I trial5 because, after disclosure of the results, the tested device was labelled as ineffective. From a meta-analytic point of view, the exclusion of a trial from the main analysis based on the missing detection of differences between groups or the subjective experience of the investigator would imply a publication bias. However, we performed a sensitivity analysis with the aim of reducing factors potentially inflating the imprecision of pooled estimates. In this analysis, we excluded the CLOSURE I and DEFENSE-PFO trials5,14 –since conclusions about specific device performance might be fair and trials with significantly shorter follow-up lengths could influence results– and applied a five-year right-censoring time to reduce inference based on limited numbers at risk. Despite these restrictions, pooled estimates were quite consistent with those computed in the main analysis.
Importantly, tPFOc cannot abolish the risk of recurrent stroke in patients with PFO and, although a significant proportion of cerebrovascular events in the interventional group may be related to suboptimal closure, causal relationships with additional factors, such as microembolism or thrombus formation on the implanted device, cannot be completely excluded2,36. In support of that, we found that the risk of TIA was comparable between groups without signals of heterogeneity. The diagnosis of TIA can be challenging compared with stroke. However, in the included trials, the occurrence of TIA was based on clinical and brain imaging data, acute cerebrovascular events during follow-up needed to be assessed by the neurologist, and atypical or unclear symptoms were not considered if not supported by further evidence. The comparable risk of TIA between groups perhaps implies the persistence in some patients who underwent tPFOc of imperceptible shunting that is not able to produce stroke but maintains some predisposition to embolisation through the interatrial septum. In addition, some anatomic characteristics, such as interatrial septal aneurysm, may confound the correlation between the significant stroke risk reduction and the lack of benefit in terms of TIA.
An important finding was that the development of atrial fibrillation is significantly higher in patients who undergo tPFOc compared with those receiving ATA. Nevertheless, the results of our analysis suggest that the net benefit of tPFOc is clear in spite of this. The review of data from the included trials shows that atrial fibrillation had onset generally limited to the early post-procedural time and in most cases was paroxysmal or treated successfully by electrical or pharmacologic cardioversion without recurrence over follow-up (59-100%). In addition, only a very small proportion of strokes in the tPFOc group had a causal relationship with atrial fibrillation. However, although the available results have acceptable follow-up length, the impact of a permanent implant on the overall risk of stroke, propensity to develop persistent atrial fibrillation and need for anticoagulation after several decades remains unexplored37. Procedural safety is further confirmed by similar incidences of major bleeding between groups. The young and low-risk profile of the enrolled patients did not lead to an excess of events in the ATA group.
The review of other major adverse events after tPFOc did not raise concerns4-7,12-14,32, with the exception of a higher incidence of pulmonary embolism in the RESPECT trial7,32, which was probably not related to the device and could be explained by the persistence of the source of embolism after successful tPFOc. Procedure- and device-related serious adverse events were very low across trials, ranging from 3.9% to 7.9%, and no differences in cumulative rates of any type of serious adverse event emerged between groups. Yet, heterogeneous reporting of information across trials was observed. Mortality over time was very low in both groups, and frequently events had a non-cardiac and non-neurologic cause.
Early observational reports suggested a possible secondary benefit of tPFOc in terms of reduction of the incidence of migraine38. However, a recent small trial has not shown a significant reduction in monthly migraine days38. Although it is mandatory to consider that in trials included in our meta-analysis the appropriate assessment of migraine was not among endpoints and the clinical subset did not necessarily comprise patients with a history of migraine, our meta-analysis indicates no benefit from tPFOc.
In aggregate, our review highlights that tPFOc can significantly reduce the risk of stroke over time compared with ATA, but the benefit seems to depend significantly on proper selection of patients in terms of clinical history and high-risk PFO characteristics. Currently, procedures performed for TIA or migraine do not present acceptable evidence-based support.
Limitations
As with any meta-analysis, regardless of aggregate data or reconstructed time-to-event data, our results depend on original investigations and share the same limitations. We did not have access to the full data set. However, we reconstructed original data according to a validated methodology having a minimal –but unavoidable– margin of imprecision25,26. The very large margin of significance/non-significance of pooled estimates as well as the strong consistency observed across multiple rigorous analyses make our conclusions robust and reliable.
In addition, the following limitations should be considered. First, with respect to the endpoints of atrial fibrillation and major bleeding, the mixed reporting across trials imposed the use of ORs as outcome measure instead of HRs. In addition, in the Gore REDUCE trial13, events were provided as atrial fibrillation or atrial flutter. Yet, the number of atrial flutters was described as being extremely low. Second, in the report of the Gore REDUCE trial13, the endpoint of TIA was not reported as an HR. We overcame this limitation by the “borrowing of strength” of multiple-outcome meta-analysis. In addition, we performed a sensitivity analysis by using counts (ORs) – reported for all the trials; results did not change. Third, in the CLOSURE I trial5, atrial fibrillation and major bleeding were shown according to per-protocol analysis. We handled denominators according to as-randomised values to be consistent with the other trials that presented results according to intention-to-treat analysis. Fourth, in the subgroup analyses of the CLOSE and Gore REDUCE trials12,13 (<44.6 versus ≥44.6 and ≤45 versus >45, respectively), the age cut-off differed trivially from the one we used (<45 versus ≥45), but the impact is considered insignificant. Moreover, with respect to subgroup analyses, in the CLOSE trial12 data were shown as any shunt plus atrial septal aneurysm, thus were not considered in our study, while in the DEFENSE-PFO trial14 no result was presented according to major clinical subgroups, thus data could not be pooled. Finally, in the DEFENSE-PFO trial14, atrial fibrillation, major bleeding, and migraine were not shown.
Conclusions
Compared with ATA, tPFOc significantly reduces the risk of stroke at long-term follow-up. However, no difference was observed in terms of TIA. The risk of atrial fibrillation is higher after tPFOc but generally early and transient. Major bleeding and migraine are comparable with ATA.
| Impact on daily practice Currently, the European Stroke Organisation indicates tPFOc only in patients with cryptogenic stroke and PFO with high-risk features, while the American Heart Association/American Stroke Association guidelines consider tPFOc a viable alternative to ATA only in patients with PFO and recurrent deep vein thrombosis. This meta-analysis shows that accumulated evidence from randomised clinical trials is robust enough to prove a significant stroke risk reduction after tPFOc compared with ATA in patients with history of cryptogenic stroke confirmed by brain imaging. The safety profile of tPFOc is good overall but associated with a higher risk of atrial fibrillation, though mostly paroxysmal or successfully cardiovertible and with onset limited to the first period. No advantage of tPFOc in terms of TIA and migraine was observed. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Conflict of interest statement
All the authors declare no support from any organisation for the submitted work, no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, and no other relationships or activities that could appear to have influenced the submitted work. D. Giacoppo has received grants from the European Association of Percutaneous Cardiovascular Interventions (EAPCI). R. Byrne has received lecture fees from B. Braun Melsungen AG, Biotronik and Boston Scientific, and institutional research grants from Boston Scientific and HeartFlow. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
The complete list of references can be found in the online version of this paper.
Supplementary data
Supplementary Appendix 1. List of included trials.
Supplementary Appendix 2. Supplementary methods.
Supplementary Figure 1. Flow diagram.
Supplementary Figure 2. Trial sequential analysis after switching the order of addition of two simultaneously disclosed trials, Gore REDUCE and CLOSE.
Supplementary Figure 3. Sensitivity analysis of TIA.
Supplementary Figure 4. Risk of migraine.
Supplementary Figure 5. Bias assessment across the included trials.
Supplementary Table 1. PRISMA checklist.
Supplementary Table 2. Literature search.
Supplementary Table 3. Key eligibility criteria across the included trials.
Supplementary Table 4. Main clinical and anatomic characteristics by group.
Supplementary Table 5. Main clinical and anatomic characteristics by trial.
Supplementary Table 6. Antiplatelet and anticoagulant medications in patients assigned to tPFOc and ATA across the included trials.
Supplementary Table 7. Primary endpoints and definitions of stroke across the included trials.
Supplementary Table 8. Ischaemic stroke across trials and follow-up time points.
Supplementary Table 9. Main adverse cardiovascular events other than atrial fibrillation and major bleeding across the included trials.
Supplementary Table 10. Evaluation according to GRADE of the overall reliability of the conclusions provided.
To read the full content of this article, please download the PDF.

