
Introduction
Prior studies have shown that percutaneous patent foramen ovale (PFO) closure increases the risk of atrial fibrillation and atrial flutter (AF)1-5. Therefore, we sought to evaluate systematically the incidence of new-onset AF post percutaneous PFO closure and to determine the risk of stroke associated with new-onset AF.
Methods
Electronic databases were searched for randomised trials that compared percutaneous PFO closure versus control for patients with cryptogenic stroke or migraine. Two independent investigators (A.Y. Elgendy and M.K. Mojadidi) extracted the data on the clinical outcomes of interest. New-onset AF was categorised into: early-onset (defined as onset occurring ≤45 days post PFO closure) and late-onset (defined as AF occurring >45 days post PFO closure). The 45-day cut-off was chosen since this was the longest follow-up period defined as “early-onset AF” in the trials4. The primary analysis was performed using the DerSimonian and Laird model for random effects summary risk ratio (RR) (PROSPERO 2017 CRD42017082640).
Results
Eight trials with 3,924 patients were included. The mean age was 45 years and 60% were female. AF with no documented recurrence was reported in 76% (59/78 patients) in the PFO closure group: these episodes resolved either spontaneously or with cardioversion (chemical or electrical) and had no documented recurrence.
At a mean follow-up of 2.8±1.7 years, the weighted mean incidence of AF in the PFO closure group was 3.2% (95% CI: 1.8-5.0%) versus 0.47% (95% CI: 0.15-0.91%) in the control group (p<0.001). PFO closure was associated with a higher risk of new-onset AF using random effects (RR: 4.30, 95% CI: 2.40-7.90, p<0.001, I2: 0%), and fixed effects using Peto’s method (OR: 3.87, 95% CI: 2.50-5.90, p<0.001) (Figure 1A), with “high” quality of evidence. Sensitivity analysis demonstrated a higher incidence of new-onset AF post PFO closure after excluding the trials that used the STARFlex device (NMT Medical, Boston, MA, USA) (RR: 3.50, 95% CI: 1.70-7.10, p=0.001, I2: 0%), but the risk was not increased when limited to the trials using the AMPLATZER™ PFO Occluder (St. Jude Medical, St. Paul, MN, USA) (RR: 2.20, 95% CI: 0.91-5.40, p=0.08, I2: 0%). PFO closure was associated with a higher incidence of early-onset AF versus late-onset AF (2.9% [95% CI: 1.4-4.8%] versus 0.52% [95% CI: 0.003-1.6%], p=0.001) (Figure 1B).
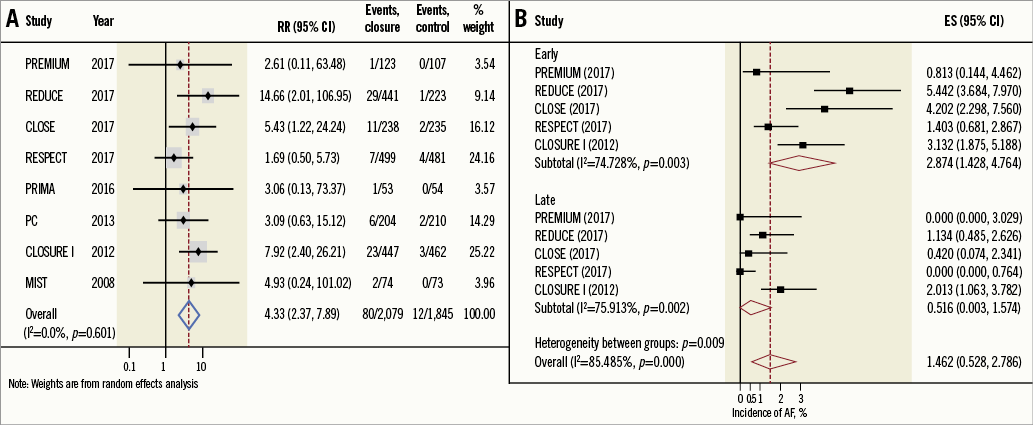
Figure 1. Relative risk and incidence of onset for AF. A) Summary random effects relative risk for AF. B) Pooled incidence of early-onset AF versus late-onset AF. ES: effect size; RR: relative risk
At a mean follow-up of 2.6 years, the pooled incidence of stroke related to new-onset AF in the PFO closure group was 0.1% (5/1,841 patients [95% CI: 0.001-0.38], p=0.91). At a mean follow-up of 2.4 years, the pooled incidence of stroke related to new-onset AF in the control group was 0.09% (4/1,387 patients, 95% CI: 0.001-0.43, p=0.87).
Discussion
This comprehensive meta-analysis of eight randomised trials including 3,924 patients who underwent percutaneous PFO closure demonstrated a fourfold increased risk of new-onset AF when compared with control. The overall incidence of new-onset AF was low. The risk of early-onset AF was significantly higher than late-onset AF. Over three fourths of device-associated AF events resolved (spontaneously or with cardioversion) without recurrence. The pooled incidence of stroke presumably caused by device-associated AF in the PFO closure group was 0.1%.
Catheter manipulation and wires crossing through the left atrium during closure device deployment can trigger AF. Certain closure devices have been linked to a higher incidence of AF by local stretch or septal irritation during and after device deployment5. In a network meta-analysis of 2,963 patients with cryptogenic stroke, new-onset AF was more pronounced with the STARFlex device (RR: 7.67), than with the AMPLATZER PFO Occluder or HELEX® device (W.L. Gore & Associates Inc., Flagstaff, AZ, USA)5. In this meta-analysis, there was an increased risk of AF across different closure devices, even after excluding the STARFlex device, but this risk was not observed when limited to the trials using the AMPLATZER PFO Occluder. The GORE® Cardioform Septal Occluder was associated with a significant increased risk of AF in the REDUCE trial4.
A major diagnostic dilemma with cryptogenic stroke is the potential presence of occult AF that can lead to recurrent stroke. Unlike primary AF, a definite benefit from long-term anticoagulation for secondary AF due to a device implantation is debatable. Prolonged monitoring with an implantable cardiac monitor significantly increases the rate of occult AF detection compared with a conventional 24-hour monitor in cryptogenic stroke; however, the rate of AF detection was not significant with age ≤65 years6. By definition, cryptogenic stroke occurs in otherwise healthy people; thus, occult AF is unlikely to be a major contributor to stroke without the presence of AF symptoms or documentation by electrocardiogram monitoring. Since prolonged monitoring was not part of the PFO trials protocol, the actual risk of AF may be higher. In the CLOSE trial3, anticoagulation was eventually discontinued in 70% of patients with device-associated AF. In this meta-analysis, the majority of AF episodes were detected periprocedurally, suggesting that AF is most likely new-onset and procedure-related, and <25% of the patients had persistent AF, which could be either primary AF or device-related (therapeutic failure) AF. A proposed algorithm for the management of AF post PFO closure is shown in Figure 2.
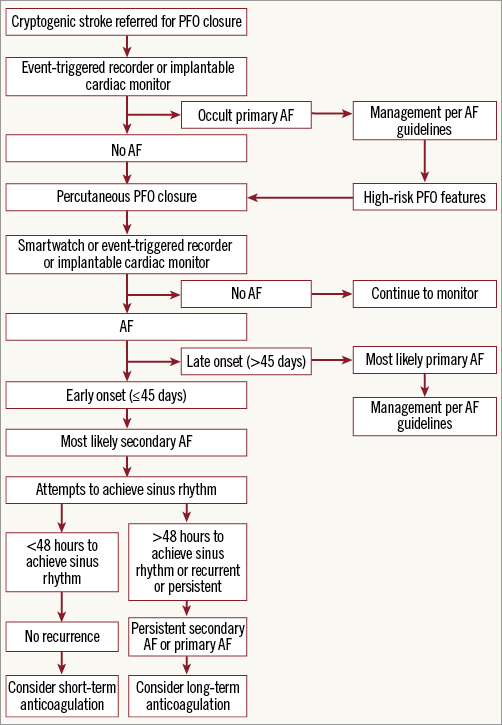
Figure 2. Proposed algorithm for the management of post-PFO closure AF.
Limitations
Lack of patient-level data limits better understanding of the effect of baseline medications (including nodal blocking agents) and demographics of patients who developed AF and the potential contributing factors to AF, including CHA2DS2-VASc score, prior occult AF, residual shunting, and enlarged left atrium. The individual trials defined cryptogenic stroke after excluding AF based on short-term conventional monitoring or an electrocardiogram; thus, some ischaemic stroke patients may have been mislabelled as cryptogenic. However, the overall incidence of follow-up AF in the included trials was less than previously reported6.
Conclusion
PFO closure is associated with an increased risk of new-onset AF. However, device-associated AF usually occurs early (≤45 days), is transient with no documented recurrence, and rarely causes a stroke.
| Impact on daily practice PFO closure is associated with increased risk of new-onset AF. The majority of device-associated AF incidences occurred early, were transient with no documented recurrence (76%), and only 0.1% of patients randomised to a device had stroke presumably caused by device-associated AF. |
Conflict of interest statement
J.M. Tobis has served as a consultant for St. Jude Medical and W.L. Gore, as a co-investigator of the RESPECT trial, on the steering committee of the PREMIUM trial, and as a proctor for Cardiac Dimensions. B. Meier has received speaker fees from Abbott, and is a co-primary investigator of the PC and PRIMA trials. The other authors have no conflicts of interest to declare.

