Abstract
Non-surgical closure of the patent foramen ovale (PFO) has been possible for 40 years and proved safe in probably a million cases performed worldwide. Nonetheless, indications are still restricted as only a few are supported by randomised data. Paradoxical embolism through a PFO causes stroke, myocardial infarction, and visceral or peripheral ischaemia. The PFO is a likely mediator of migraine, diving or high altitude sickness, dyspnoea, and sleep apnoea problems. As untoward effects of a PFO are rare and spaced widely timewise, large cohorts and long follow-ups are required to prove unequivocally that PFO closure is beneficial and appropriate in comparison to no treatment or medical therapy. The most compelling respective randomised data have been gathered so far in the realm of secondary prevention of cerebral attacks and migraine. Invariably they showed a numerical advantage of PFO closure with significant difference in sub-analyses. Evidence-based medicine carries a danger of underutilising valuable therapies while accumulating further data. PFO closure is an example. Its documented innocuousness invites a more proactive reflection in upcoming guidelines. At worst, PFO closure may not convey the projected amount of benefit. This even opens the door for primary prevention in some PFOs with high-risk characteristics.
The clinical relevance of the patent foramen ovale: paradoxical embolism and migraine
Ischaemic stroke is a leading cause of morbidity and mortality in western countries, with an incidence of approximately 700,000 cases per year in the USA1. Among these, cryptogenic stroke is encountered in almost 50% of cases2. The presence of a patent foramen ovale (PFO) represents a distinctive cause of stroke with a similar causal relationship to other accepted aetiologies of stroke (e.g., atrial fibrillation, stenosis of the carotid artery). Notwithstanding, the finding of a PFO is often, misleadingly, lumped together with unknown causes of stroke summarised under the misnomer “cryptogenic” stroke or embolic stroke of undetermined source (ESUS)3 – these terms should be avoided in the presence of a PFO.
The presence of PFO and the risk of stroke is of clinical relevance in both young and elderly populations. The most frequent cause of paradoxical embolism through a PFO is the migration of a thrombus originating from the deep venous circulation of the lower body half. It has been demonstrated that the prevalence of deep venous thrombosis (DVT) and venous thromboembolism (VTE) significantly increases in the elderly4. Furthermore, the presence of DVT and VTE is independently associated with an increased risk of stroke and myocardial infarction5 – the most obvious mechanism being the presence of a PFO, the typical prerequisite for paradoxical embolism.
Despite the presence of a PFO in about 20%-25% of the population, an association between the presence of a PFO and stroke is proven not only in younger, but also in older patients2.
Paradoxical embolism due to PFO may be associated with detrimental and potentially lethal complications other than stroke, such as myocardial infarction and peripheral embolic events, and the presence of PFO has been demonstrated to be an independent predictor of death and adverse outcome in patients with major pulmonary embolism6-9.
In addition, several studies have observed a higher prevalence of PFO in patients suffering from migraine, particularly in patients with aura. An increased prevalence of migraine in subjects with PFO has also been established10,11. Percutaneous closure of a PFO has been reported to reduce migraine frequency, suggesting a causal link between PFO and migraine12-15. Suggested hypotheses are paradoxical microembolism, which may act as a potential trigger for migraine attacks, and right-to-left shunting of neurotransmitters such as serotonin gushes not neutralised in the lungs, hence triggering a particular sensor in the brain.
The evidence in favour of a percutaneous closure of PFO has been the subject of iterative debate over recent years. This is mainly due to the fact that data from randomised, controlled trials (RCTs) comparing PFO closure vs. medical therapy for secondary prevention of stroke did not meet their primary endpoint. However, as-treated and per-protocol analyses as well as several meta-analyses have reported a benefit of PFO closure over medical therapy. Extended follow-up of the Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment (RESPECT) trial16, for instance, showed a significant benefit of PFO closure over medical therapy in the intention-to-treat analysis, with a 70% reduction of recurrent cryptogenic stroke in the closure group (RESPECT Extended Follow-up Results. J. Carrol, San Francisco, CA, USA, TCT 2015).
Considering that PFO closure is a safe, technically simple, and effective procedure that can be carried out by a single operator in less than 15 minutes, it is our opinion that this intervention is underutilised, especially in a high-risk subgroup of patients who could even benefit from PFO closure for primary prevention.
Technique of PFO closure
Our preferred device is the AMPLATZER™ PFO Occluder (St. Jude Medical, St. Paul, MN, USA) (Figure 1). Alternatively, many other “AMPLATZER-like” devices can be used interchangeably (e.g., Occlutech® Figulla® Flex II [Occlutech International AB, Helsingborg, Sweden], Hyperion™ PFO Occluder [Comed BV, Bolsward, The Netherlands], GORE® HELEX® Occluder [W.L. Gore & Associates, Inc., Flagstaff, AZ, USA] and many more), while devices other than AMPLATZER-like devices have been shown to be inferior in terms of thrombus formation, embolisation or incidence of atrial fibrillation17.

Figure 1. The AMPLATZER™ PFO Occluder (typical diameter is 25 mm).
The procedure is performed under local anaesthesia, best with fluoroscopic guidance alone. Performing PFO closure with transoesophageal echocardiographic (TOE) guidance makes the procedure more expensive and laborious without adding crucial information not obtainable by fluoroscopy. It may even add some risk (e.g., oesophageal tears, additional femoral venous puncture, or clot formation in an unflushed sheath while acquiring and interpreting echocardiographic pictures).
Usually, 5,000 units of heparin are given upfront, followed by right femoral vein puncture, and introduction of a regular and normal length 0.035 inch J-tip guidewire through the puncture needle. Frequently, the wire will spontaneously pass through the PFO into the left atrium. Otherwise, a multipurpose catheter is used to direct the wire through the PFO. The delivery sheath (typically 9 French [Fr]) is advanced over the wire to the left atrium. In cases of ad hoc PFO closure without previous TOE imaging, it may be helpful to place the sheath in the right atrium, removing the sheath introducer, followed by a contrast medium injection in a left anterior oblique (LAO) view with cranial angulation (typically 10º LAO, 10º caudal). In this view – parallel to the interatrial septum – the thickness of the septum secundum and the mobility of the septum primum can be visualised and will guide sizing of the device (Figure 2). The more mobile the septum primum and the thicker the septum secundum, the bigger the chosen device should be. This will ensure correct positioning of the two discs at the price of a slightly higher rate of residual leaks at a TOE control after three to six months of follow-up.
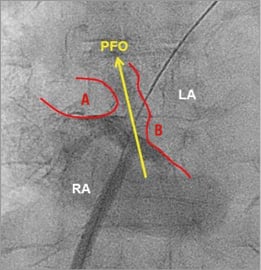
Figure 2. Fluoroscopic imaging of the patent foramen ovale (PFO). The thick septum secundum (A) is well delineated. B: septum primum; LA: left atrium; RA: right atrium
The AMPLATZER PFO Occluder device is then de-aired outside the patient’s body and introduced into the sheath. Pushing the device forward in the sheath will allow deployment of the left atrial disc in the left atrium. The sheath and the device (still attached to the pusher cable) are then pulled back as a unit until the left disc is held back by the interatrial septum. Thereafter, the right disc is deployed on the right atrial side. A push-and-pull test will confirm a stable device position, and a perfectly perpendicular position of the fluoroscopic image is chosen to perform a contrast medium injection. The typical Pacman sign should be visible, indicating a larger cranial separation of the two discs in the area of the thicker and usually well delineated septum secundum (Figure 3).
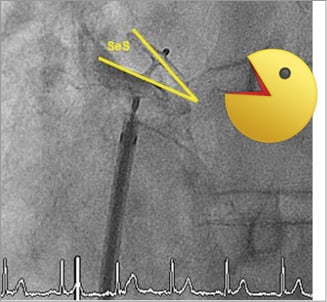
Figure 3. The so-called Pacman sign, indicating a larger cranial separation of the two discs in the area of the thicker septum secundum (SeS).
The device is then unscrewed and the final position is documented again by contrast medium injections, adjusting the projection to see the two discs again perpendicularly and completely separated.
The patient can usually leave the hospital a few hours later without any physical restrictions. Oral anticoagulation can be stopped on the day of the procedure. Single or dual antiplatelet therapy is initiated for three to six months, at which time a follow-up TOE is performed to exclude residual shunt or device thrombus. If neither is detected, antiplatelet agents can be terminated, unless another indication exists.
PFO closure and secondary prevention: current evidence
Three RCTs comparing PFO closure vs. medical therapy for secondary prevention of stroke have been published so far: Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale (CLOSURE I)18, Percutaneous Closure of Patent Foramen Ovale in Cryptogenic Embolism (PC)19, and RESPECT16.
The CLOSURE I trial18 randomised more than 900 patients to PFO closure (using the since discontinued STARFlex™ device; NMT Medical, Boston, MA, USA) or medical therapy. Medical therapy included oral anticoagulation with vitamin K antagonist or antiplatelet therapy in all three trials. Follow-up ranged from two to four years.
The PC trial19 (414 patients randomised 1:1) and the RESPECT trial16 (980 patients randomised 1:1) compared PFO closure using the AMPLATZER PFO Occluder to medical therapy.
Regarding safety, the STARFlex device which was used in the CLOSURE I trial was associated with an increased risk of non-cerebral adverse events. However, both the PC and RESPECT studies showed that PFO closure with the AMPLATZER Occluder is safe, because the occurrence of adverse events was not different compared to medical therapy. Moreover, no disabling adverse events or deaths were observed. The only difference was a non-significant increased incidence of atrial fibrillation in the device group in the PC trial (2.9% vs. 1.0%). Most of these events occurred and resolved within the first two weeks after the treatment and did not have any long-term therapeutic consequences (e.g., no need for oral anticoagulation).
The better safety profile of the AMPLATZER closure device has been confirmed in a randomised study comparing the AMPLATZER device to the STARFlex and the GORE HELEX device20.
Regarding efficacy, complete PFO occlusion with absence of residual shunt was observed in 93%-95% of patients with the AMPLATZER device. In the case of a residual shunt being diagnosed at follow-up, the risk of recurrent events is increased and a second device can be implanted.
Although all three RCTs failed to show significant superiority of PFO occlusion over medical therapy in the intention-to-treat analysis, all showed a trend towards fewer events at follow-up in the device group compared to the medical therapy group. This suggests that the three trials were not adequately powered in terms of patient numbers and follow-up duration. On the other hand, per-protocol and as-treated analyses of the RESPECT trial showed superiority of PFO closure over medical therapy16.
Moreover, a subgroup post hoc analysis of the RESPECT showed superiority of PFO occlusion compared to medical therapy in patients with large PFOs or with an atrial septal aneurysm and when compared to patients treated with acetylsalicylic acid alone (p=0.03)16. No differences were observed in comparison to oral anticoagulation with vitamin K antagonists. Therefore, the only valid alternative to PFO occlusion (which is a safe and once-in-a-lifetime intervention) is chronic life-long oral anticoagulation, which is not patient-friendly and is associated with an at least 2% annual risk of major bleeding complications.
Further evidence of the benefit of PFO occlusion for secondary prevention of stroke has been provided by different meta-analyses21,22. Kent et al performed the only pooled analysis of individual participant data from the three above-mentioned RCTs. PFO closure reduced the overall incidence of recurrent stroke and had a statistically significant effect on the composite outcome of stroke, transient ischaemic attack, and death in adjusted analyses23.
The results of the extended follow-up of the RESPECT trial were presented at last year’s TCT Meeting (RESPECT Extended Follow-up Results. J. Carrol, San Francisco, CA, USA, TCT 2015): a significant 70% reduction of recurrent cryptogenic stroke after PFO occlusion was observed during follow-up. It can be speculated that the curves further diverge, resulting in an even larger benefit of PFO closure with longer follow-up. This is supported by an observational non-randomised trial of patients randomly assigned to medical therapy or PFO closure, where very long follow-up showed a significant reduction in paradoxical embolism and a significant 40% reduction in death after PFO closure14.
From the initial RESPECT data we learned that the number needed to treat (NNT) to prevent one stroke was 25 within the first five years. Making a conservative assumption, this would lead to a NNT of five in patients with a life expectancy of 25 years (e.g., 60-year-old patients) and an even lower NNT in patients receiving PFO closure at a younger age. Since thrombus formation increases with increasing age, it can be speculated that the NNT is most probably even smaller.
Overall, the incidence of device-related events was extremely low in the AMPLATZER trials (no intraprocedural strokes, no device embolisations, no device thrombosis, no device erosions, 0.9% vascular complications).
Which patients could benefit from primary PFO occlusion?
As outlined above, PFO closure is the therapy of choice in patients with previous stroke.
Besides, PFO occlusion should be considered as primary prevention in some high-risk categories of patients. These risk factors can be PFO-related or patient-related. Some anatomical findings qualify a PFO as more dangerous for paradoxical embolism (association with atrial septal aneurysm, Eustachian valve, and Chiari network or the presence of a large spontaneous shunt)24, and may justify primary closure before an event occurs.
Although the PRIMA randomised trial showed that, in patients with refractory migraine with aura and PFO, PFO closure did not significantly reduce overall monthly migraine days15, post hoc analyses of the PRIMA trial in fact showed that migraine attacks preceded by an aura and migraine with aura days were significantly reduced in the device group compared to controls. Patients with complete cessation of migraine were 10% in the PFO closure group and none among controls (p=0.05). Looking only at patients freed of migraine with aura, the difference was even more significant (p=0.004). Therefore, since patients suffering from migraine (especially with aura symptoms) and PFO have a high probability of experiencing an improvement of their symptoms after PFO closure, they could, in our opinion, represent an adequate target for PFO closure for primary prevention of paradoxical embolism. Besides the chance of improvement of migraine, patients would indeed also be protected against paradoxical embolism.
A similar association of PFO with other illnesses has been described and PFO closure should be considered in these patients: patients suffering from high-altitude sickness, from sleep apnoea, or from platypnoea orthodeoxia syndrome. Other patient factors that may qualify for PFO closure outside of secondary stroke prevention are: persons with high-risk activities (e.g., professional divers, brass musicians, or weightlifters) and persons at high risk of venous thrombosis (e.g., frequent fliers). A simple screening transthoracic echocardiography bubble exam could identify persons at high risk, i.e., those with a large (dangerous) PFO, not requiring TOE for detection.
Guidelines and current recommendations
European and American guidelines25,26 currently do not reflect the recent evidence supporting PFO occlusion. The European Stroke Organisation guidelines of 2008 only consider PFO closure in patients with cryptogenic stroke and a high-risk PFO (defined by the presence of an atrial septal aneurysm, Eustachian valve, or Chiari network), or in patients with PFO and recurrent events (both class IV recommendations). The American Heart Association/American Stroke Association guidelines consider PFO closure in patients with stroke or transient ischaemic attack (TIA) only in the presence of clinical evidence of deep venous thrombosis (DVT, class IIb, level C), while, in the absence of DVT, PFO occlusion is not recommended.
We recently proposed an updated and a more patient-tailored approach for PFO closure (Table 1)27.

Further elaborations on these points are:
– In the presence of a PFO in all patients with stroke or TIA, PFO closure should be carried out to eliminate at least one of the potential stroke causes, even in the presence of other potential causes.
– PFO closure should be avoided only in the presence of another concomitant indication for oral anticoagulation or in the presence of anatomical contraindication to occlusion. In this last circumstance, oral anticoagulation is recommended.
– PFO closure should also be considered for primary prevention in patients at high risk of paradoxical embolism due to the tendency for venous thrombosis, vocational or recreational activities fostering right to left shunts, in the presence of high-risk PFO, or those who can expect a collateral benefit (e.g., patients suffering from migraine, sleep apnoea, etc.).
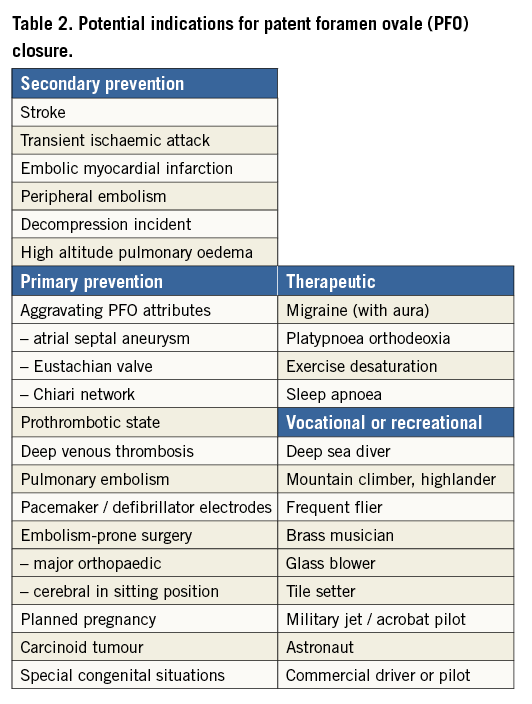
Table 2 summarises the potential indications for PFO closure.
Conclusions
Percutaneous PFO occlusion is a simple, safe, and effective treatment which can be beneficial for a large number of patients. It is a typical “once-in-a-lifetime” intervention that can effectively protect against dangerous events, such as stroke, myocardial infarction, or other systemic embolism.
European and American guidelines do not reflect the current evidence and should therefore be updated.
In our opinion a more proactive role in PFO closure for secondary prevention (and even for primary prevention in a specific subset of patients) is mandated.
Conflict of interest statement
F. Nietlispach received fees for proctoring from St. Jude Medical. B. Meier received fees for proctoring and research grants to the institution from St. Jude Medical. The other authors have no conflicts of interest to declare.

