
In this issue of EuroIntervention, a multi-authored position paper on the management of patients with patent foramen ovale (PFO) and “left circulation thromboembolism” (ischaemic stroke or systemic non-cerebral embolism) is published1.
This represents an enormous contribution by an impressive group of internationally known investigators – independent contributors and representatives of European professional organisations. They forged a consensus statement on the evaluation, diagnosis, and treatment of these patients. I can imagine the fractious conversations. With any multi-authored publication, internal disagreements become quieted so that the final consensus document appears as settled science. The risk is that the positions become fixed in people’s minds because, as Margaret Thatcher noted, “Nothing is more obstinate than a fashionable consensus”. Consensus requires compromises; however, a reviewer has an unopposed opportunity to point out where they would not have compromised. I appreciate the opportunity to be so indulged.
First, a point of praise. I agree that better terms are needed for PFO-related strokes – “cryptogenic stroke with PFO” is a product of PFO’s emergence as a plausible stroke mechanism only after the influential classification systems were developed. These systems (such as TOAST2 and ASCOD3) were designed to categorise strokes into mechanistic aetiologies for research. Given the paucity of information on PFOs at the time, PFO-related stroke was not included as a category in its own right. However, PFOs were consistently found in significantly elevated numbers of patients with strokes that were otherwise unexplained – thus, “cryptogenic stroke with PFO.” This, however, belittles our confidence in the diagnosis and seems unconvincing. Cardiogenic stroke from atrial fibrillation is not similarly afflicted with hesitation. We do not diagnose a “cryptogenic stroke with atrial fibrillation”, even though embolisation of a left atrial appendage thrombus is rarely seen. Almost all stroke diagnosis is probabilistic. I favour a tiered category of definite, probable, and possible PFO-related stroke, informed by predictive models such as the Risk of Paradoxical Embolism (RoPE) score4 and interpreted with clinical judgement based on biological plausibility and other collateral information. However, the further we stray from solid science and rely on experience and experts, the more we must be prepared to yield when data emerge, even if they challenge what is considered axiomatic. Cue Thomas Huxley: “The great tragedy of Science [is] the slaying of a beautiful hypothesis by an ugly fact”5. More on this below.
In the emerging PFO science, there are three risk dimensions – to be considered separately. The authors refer to the “axes of evaluation” but only describe two axes – and hint at a third. Risk 1 is PFO relatedness to the index event. Because of the high prevalence of PFO in the general population (~25%), discovering a PFO in a cryptogenic stroke patient is only the beginning of the process of determining if the PFO is incidental or pathogenic. The most widely used tool for distinguishing between good and bad PFOs is the RoPE score although, as was pointed out, validation of the RoPE model has only been carried out with small populations6,7. Risk 2 is the likelihood of stroke recurrence. Some variables have been found prognostically useful, such as a hypermobile interatrial septum (the misnomered “atrial septal aneurysm”). Some variables have different predictive directions – e.g., young age increases the likelihood of Risk 1 (PFO relatedness) but decreases Risk 2 (stroke recurrence). The third axis of risk, or Risk 3, refers to the likelihood that an individual patient will benefit from closure. Almost all clinical trials have heterogeneity of treatment effect8 – i.e., some patients benefit, some do not, and some are harmed. Heterogeneity depends, in part, on the baseline risk of the outcome (Risk 2 here) but it is not identical to that risk. This consensus document conflates these two risk categories – i.e., if there is a high risk of recurrence (Risk 2), then there is a greater benefit of treatment (Risk 3). This remains to be shown.
The RoPE score is “agnostic” about PFO features. It is criticised for that in this paper. The authors assert to know how to distinguish the high-risk PFOs from the low-risk ones. However, by not conditioning the RoPE score on PFO features, a curious finding emerged. Small shunts have a higher risk of recurrence than large ones and this is true only in probable pathogenic PFOs9 (Table 1).
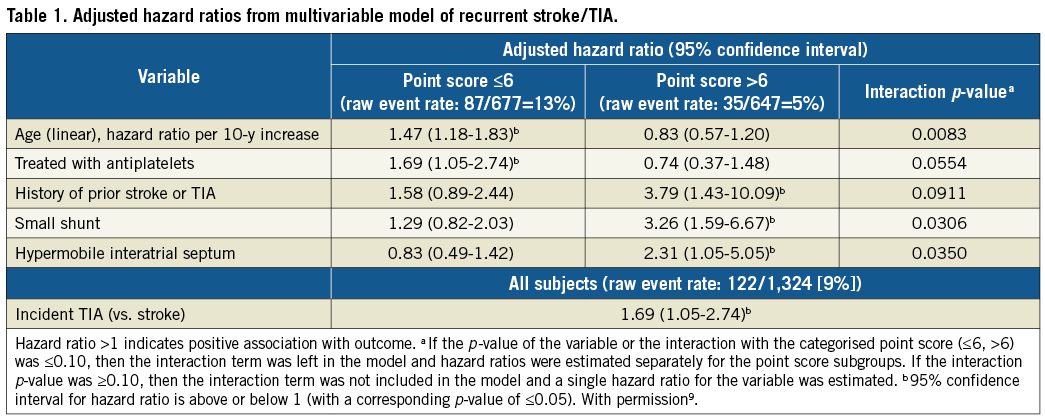
Here is Huxley’s sword. This finding is counterintuitive and not widely accepted. The paper assumes that large shunts are “high risk” (Risk 1 or 2?). Nevertheless, the small-shunt-is-risky paradox may be true. One explanation is that there may be a PFO-related stroke mechanism separate from paradoxical embolism, such as in situ thrombus. This should be more common in smaller PFOs where slow shunt flow results in blood stasis and an occasionally opened tunnel. A second explanation might be that small shunts found on conventional echoes (upper extremity injections) are markers for larger shunts that would have been found if only the contrast bolus had come via the inferior vena cava – like most paradoxical embolisms. So, it may be an artefact of our diagnostic tests. In any case, shunt size, as measured with bubble counts, may not be a reliable marker of “risk,” even though it is intuitively compelling.
Much is made of the subgroups, tested one at a time, within the PFO trials (SupplementaryFigure4, SupplementaryFigure5)1. Special emphasis is placed on “high-risk” PFO features such as large shunt size and a hypermobile septum and the suggestion of a greater benefit from PFO closure in patients who harbour them. Subgroup differences, even “significant” ones, should stimulate testable hypotheses, not be embraced as proof of relationships. The DEFENSE-PFO trial and its powerful treatment effect (but with an enormous confidence interval) is identified as evidence of the success of patient selection by only including patients with “high-risk” PFOs. However, given that “low-risk” PFOs were excluded, then the DEFENSE-PFO trial must be interpreted as silent on differential treatment effects based on PFO characteristics. Comparing outcome rates across studies is precarious. I urge caution in any treatment decisions based on purported “high-risk” PFOs. The danger is not that we will overtreat the “high-risk” ones, but the inescapable corollary is that treatment should be withheld from the “low-risk” ones. “Low-risk” patients were included in RESPECT and REDUCE – and both were positive trials. Are those who advocate for treatment decisions based on potentially unreliable echo characteristics now willing to randomise stroke patients with probable PFO-related index strokes to no closure because nine bubbles were seen in the left atrium rather than 11?
I began with a cautionary conservative British voice and end with a liberal American one. Franklin Roosevelt warned that “there are as many opinions as there are experts”. These experts surely have more opinions than they could express in this paper but their contribution here is substantial. They offer an enormously valuable review of the field and some guidance going forward. This expert has offered some more opinions but the strongest one is gratitude for their work.
Conflict of interest statement
D. Thaler is a member of the Steering Committee of the RESPECT Trial for Abbott, and received prior grant funding from NIH: UL1 RR025752 and R01 NS062153. S. Gans has no conflicts of interest to declare.

