Abstract
Patent foramen ovale (PFO) is implicated in the pathogenesis of a number of medical conditions but to date only one official position paper related to left circulation thromboembolism has been published. This interdisciplinary paper, prepared with the involvement of eight European scientific societies, reviews the available evidence and proposes a rationale for decision making for other PFO-related clinical conditions. In order to guarantee a strict evidence-based process, we used a modified grading of recommendations, assessment, development, and evaluation (GRADE) methodology. A critical qualitative and quantitative evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk/benefit ratio. The level of evidence and the strength of the position statements were weighed and graded according to predefined scales. Despite being based on limited and observational or low-certainty randomised data, a number of position statements were made to frame PFO management in different clinical settings, along with suggestions for new research avenues. This interdisciplinary position paper, recognising the low or very low certainty of existing evidence, provides the first approach to several PFO-related clinical scenarios beyond left circulation thromboembolism and strongly stresses the need for fresh high-quality evidence on these topics.
Introduction
Patent foramen ovale (PFO) is implicated in the pathogenesis of a number of medical conditions. However, the high prevalence of a PFO in the normal population (20-30%) implies that PFO can often be an incidental finding rather than a causative one. To help clinicians with decision making, the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Scientific Documents and Initiatives Committee invited eight European scientific societies and international experts to develop interdisciplinary position statements on the management of PFO, based on systematic assessments of the literature.
A previous position paper has been published addressing issues related to cryptogenic thromboembolism1,2. The present paper reports the approach to patients with PFO and decompression sickness, desaturation syndromes, migraine, and other clinical presentations.
Methods
To guarantee a strictly evidence-based process, position statements were developed using modified grading of recommendations assessment, development, and evaluation (GRADE) methodology (http://gdt.guidelinedevelopment.org/app/handbook/handbook.html), and by answering population-intervention-comparator-outcome (PICO) questions and non-PICO questions. A detailed review of the methodology employed can be found in Supplementary Appendix 1 and in an appendix of the previously published first part of this position paper1. Systematic reviews and statistical analysis were performed by a dedicated evidence synthesis team. A detailed insight and discussion of each section and the most important paragraphs can be found in Supplementary Appendix 2.
DECOMPRESSION SICKNESS
Decompression sickness (DCS) is a complex condition triggered by the trapping of gas emboli in vessels and tissues, which can result in a wide range of acute clinical scenarios, from mild to severe, with possible persistent disability or death. DCS occurs when a person moves from a higher pressure to a lower pressure area, such as a rapid ascent at high altitude or a rapid ascent from depth (compressed air work or diving).
A PFO can allow paradoxical embolisation of venous gaseous emboli (VGE) when there is a rise in right heart pressures due to pulmonary gas embolism or physical exercise; however, in large PFOs with spontaneous right-to-left (R-T-L) shunts, paradoxical VGE can also occur without other provocation3,4. Mild embolism may cause subclinical lesions, with still unknown late consequences5,6,7,8,9.
The risk of DCS from diving is difficult to estimate, but an incidence up to approximately 1.5% has been reported10. In divers, the association between PFO and DCS is supported by retrospective case-controlled epidemiological studies, mechanistic studies and association studies. In our meta-analysis of four correlation studies comparing the prevalence of R-T-L shunts in patients with and without DCS, we found an odds ratio (OR) of 5.63 (95% CI: 3.14-10.09) for R-T-L shunts in patients with DCS11,12,13,14 (Supplementary Figure 1).
The occurrence of altitude DCS is lower and is decreasing over time. High-altitude military pilots with long flights in a hypobaric environment (i.e., U2 plane pilots) may have short-term and long-term complications15,16,17 but there are no studies published about correlation with cardiac defects. Therefore, the role of PFO in individual cases of altitude DCS can remain elusive6,7,18,19.
IS IT CLINICALLY POSSIBLE TO ESTIMATE THE PROBABILITY OF A CAUSAL RELATIONSHIP BETWEEN A PFO AND DECOMPRESSION SICKNESS?
Determination of a causal role of PFO in DCS is difficult and should take into account that systematic and prospective evaluations of PFO-associated DCS are lacking; considerations can only be based on case reports, retrospective and mechanistic studies. Therefore, an individual assessment is mandatory. PFO-related DCS can produce earlier and more abundant VGE arterialisation but its role should be weighed against other individual factors that affect VGE production and trapping (dive/flight characteristics, physiological characteristics of tissues and factors that influence the threshold of “VGE tolerance”). Therefore, a technical analysis of the pre-decompression and decompression phase characteristics of each particular case is necessary20. In professional divers suffering from PFO-associated DCS, PFO size has been found to be a predictor of recurrence21,22. The main characteristics which can be considered are summarised in Supplementary Table 1, with position statements in Supplementary Table 2.
DIAGNOSTIC WORKUP
Patients with a history of DCS should have a thorough workup to identify factors that may have led to the occurrence of DCS. DCS has multiple and non-specific clinical manifestations (Supplementary Table 3),23; there are no imaging or laboratory test patterns which are unequivocal for DCS. Therefore, the diagnosis of DCS should be made by an experienced hyperbaric or aerospace physician according to the characteristics of the exposure, symptoms and the absence of other causes. VGE detected by echocardiography in patients with suspected DCS reinforces the diagnosis24.
High-resolution computerised tomography (CT) scanning and pulmonary function testing with bronchial provocation testing can exclude alveolar barotrauma but should not delay prompt recompression treatment of DCS.
When the diagnosis of DCS is unlikely, it may be unnecessary to begin secondary prevention workup even if a PFO is known to be present. In cases of DCS where no obvious risk factors for DCS can be identified or in activities with a high but non-modifiable risk of DCS, PFO screening should be considered part of the diagnostic workup.
PFO screening should be carried out at experienced sites, employing the previously published diagnostic approach1,2 to minimise false-negative tests, which could increase DCS risk during subsequent activities due to a false sense of security25,26.
When a clear, modifiable cause can be identified (e.g., diving outside acceptable decompression limits), or when more than two risk factors known to increase the risk for DCS are present (e.g., dehydration; heavy exercise at depth or at height; diving while cold near the end of the dive causing peripheral vasoconstriction; alcohol consumption), screening for PFO is not generally recommended27.
Figure 1 displays the recommended stepwise approach to DCS.
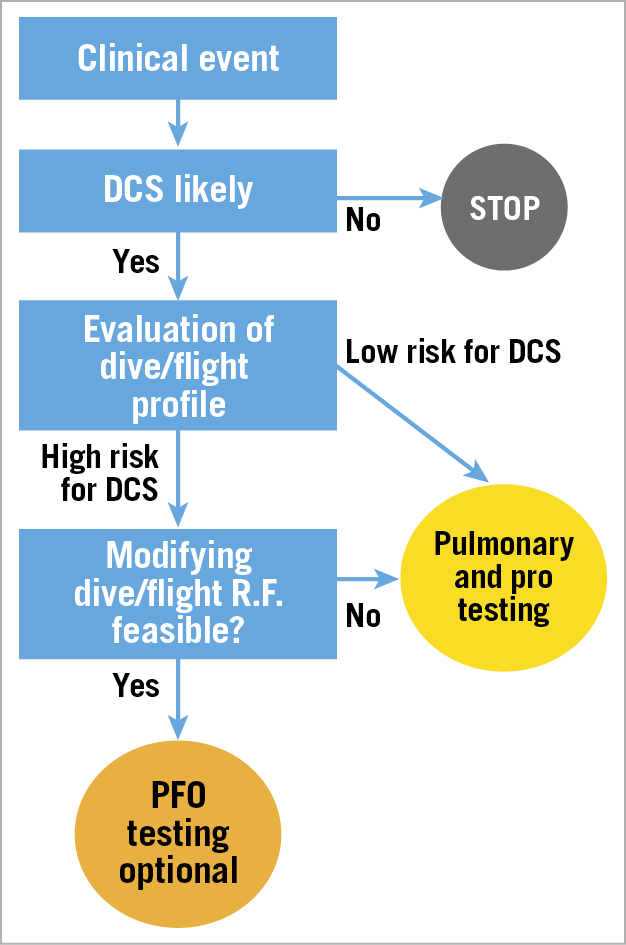
Figure 1. Flow chart depicting strategy for investigation after DCS. R.F.: risk factors
SECONDARY PREVENTION
There are no published randomised studies comparing PFO closure to behavioural prevention of DCS. Moreover, limited observational evidence in divers is available and no data are available for aircrews. However, on any occasion, inhibiting the production of VGE has the potential to prevent further DCS, irrespective of the presence of a PFO28,29. This can be achieved by: a) modifying the individual’s lifestyle and personal physiologic characteristics (smoking and alcohol consumption, body weight, ensuring adequate hydration pre and post dive); b) avoiding those technical dive or flight factors that have caused abnormal VGE production; and c) reducing the inert gas saturation of tissues before decompression by breathing high concentrations of oxygen before the ascent (Supplementary Table 4).
Nonetheless, there are certain categories of aircrew or diver for whom performing conservative flights or dives is not a realistic option27,30. In these people, PFO closure may be proposed based on observational data suggesting that PFO closure is associated with reduced DCS incidence in divers5 and prevents arterialisation of VGE31,32,33. However, since recurrent DCS has also been observed after PFO closure in some studies, it should be remembered that diving may be a cause of DCS even without a PFO33,34,35. Current recommendations are that diving should be resumed only in the presence of a sealed PFO30,36. In the absence of complete closure post procedure, divers should not be allowed to return to “unrestricted” diving and should only make low-risk dives.
Figure 2 displays the treatment algorithm developed by this task force; Supplementary Table 2 displays the position statements.
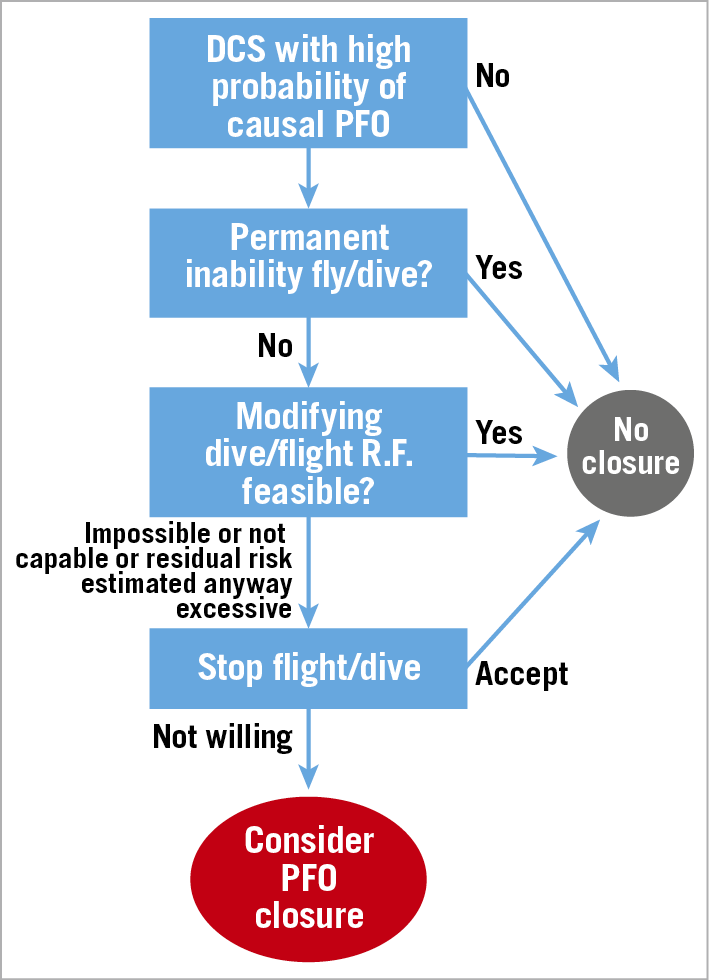
Figure 2. Flow chart for therapeutic decision making for DCS. R.F.: risk factors
IS A PRIMARY SCREENING OR PREVENTION ADVISED?
No evidence-based statements can be formulated regarding PFO closure as primary prevention of DCS.
General lifestyle and behavioural changes and technical procedure adherence are usually indicated in both divers and flying crews.
Based on the mismatch between the high prevalence of PFO and low incidence of DCS, it is suggested that primary screening for PFO should not be carried out on a routine basis in either divers or conventional altitude pilots30,36,37,38.
However, in professional divers, primary screening for PFO can be foreseen in accurately selected cases with high-risk work activity, in order to evaluate the possibility of a primary percutaneous closure. On the same basis, primary screening for PFO could be carried out in select military pilots performing intensive very high-altitude flight activities16,17. However, primary PFO closure in pilots should be weighed against the possibility of disqualification from flight activity.
When a PFO is an incidental finding in pilots or divers with no history of DCS, no restriction in conventional altitude flights is required, while recreational divers should be counselled by an experienced diving physician either to stop diving, or to undertake only low-risk profile dives. PFO closure indications should always be considered in conjunction with an experienced diving or aerospace physician.
PRACTICAL SUMMARY 1: DECOMPRESSION SICKNESS
WHAT TO DO
- PFO screening in DCS cases with no obvious risk factors or with high but non-modifiable risk for DCS
- After a DCS, primarily prevent bubbles with behavioural and technical (B&T) changes
- If B&T changes are not possible or not effective, PFO closure can be proposed with shared decision making underscoring the lack of evidence
- Resume unrestricted activity only after confirmed PFO sealing post intervention
WHAT NOT TO DO
- Primary PFO screening
- Deny conventional flight or diving after incidental finding of PFO
- High-risk recreational dives after incidental finding of PFO
- Propose PFO closure if B&T changes can be made and are effective
MIGRAINE
Migraine is a common disorder which affects approximately 12% of the general population (4-9% of men and 15-17% of women between 20 and 64 years of age39) and is often disabling40. It is estimated that 1-4% of the population meet the criteria for chronic migraine41,42. In the general population, it is estimated that the prevalence of migraine with aura ranges from 1 to 4% in men and 3 to 10% in women43.
Position statements are summarised in Supplementary Table 5.
IS PFO ASSOCIATED WITH MIGRAINE? WHAT ARE THE UNDERLYING MECHANISMS?
The association between PFO and migraine has been suggested by a higher prevalence of PFO in those with migraine, especially among those with aura, than in the general population44,45,46,47,48,49,50,51 and by the finding of incidental improvement in migraine in patients who have undergone percutaneous closure of the PFO for other reasons52,53. Moreover, the high prevalence of migraine attacks in some disorders wherein atrial or pulmonary shunts exist54,55 would suggest a pathogenic role of R-T-L shunts.
However, the association between migraine and PFOs varies considerably across heterogeneous populations46,56,57,58,59,60.
The most plausible electrophysiological substrate of headaches and aura symptoms is cortical spreading depression (CSD)61,62 which, in this case, would be triggered by paradoxical cerebral thromboemboli45,47,49,61,63,64,65,66,67 and/or the direct passage of metabolites into the systemic circulation, also possibly caused by the release of active metabolites from platelets activated by shear stress in the PFO, resulting in irritation of the trigeminal nerve and the brain’s vascular network67,68.
IS IT CLINICALLY POSSIBLE TO ESTIMATE THE PROBABILITY OF A CAUSAL RELATIONSHIP BETWEEN A PFO AND MIGRAINE?
In some retrospective and prospective observational studies, a higher prevalence of an atrial septal aneurysm (ASA)69 and larger PFO sizes in subjects with migraine with aura has been reported60,70. Also, the number of bubbles crossing the PFO, as detected by contrast transcranial Doppler (cTCD), has been found to correlate with the severity and frequency of attacks among migraineurs with aura in other studies45,56. However, the results of other studies do not support an association between the frequency of migraine attacks and PFO characteristics56,57,58.
In patients with previous stroke, an association between PFO and migraine has been reported47, and percutaneous closure has been shown to be more effective at reducing the frequency and severity of migraine attacks than in patients without cerebrovascular disease71,72.
Older age seems to be associated with an absence of any relationship between PFO and migraine59,60.
TREATMENT
To date, three randomised studies73,74,75 and three meta-analyses72,76,77 have addressed the issue of percutaneous closure as therapy for migraine. We performed an updated meta-analysis of randomised and observational studies to support the position statements in this document.
Observational studies yielded a statistically significant improvement in migraine, albeit with marked inconsistency between studies, whereas individual randomised clinical trials (RCTs) and their meta-analyses failed to demonstrate any statistically significant difference in primary outcomes, responder rates or complete migraine resolution. On the other hand, a meta-analysis of secondary endpoints revealed a statistically significant reduction in migraine attack frequency and duration. Also, subgroups of patients with aura and patients with cerebrovascular disease experienced statistically significant improvement in migraine with PFO closure, when compared to medical therapy (Supplementary Figure 2-Supplementary Figure 4).
One thing to be considered is that, according to GRADE methodology, the certainty of effects was judged severely, implying that a number of limitations need to be addressed in future studies (Supplementary Table 6). Moreover, it is possible that the neutral primary results of PFO closure studies may be due to the inclusion of patients without a causative PFO2. Additionally, the choice of migraine study endpoints is problematic, being largely arbitrary78. Therefore, further RCTs are necessary to obtain satisfactory certitude of effects.
Supplementary Table 7 shows the GRADE table for the treatment of migraine and Figure 3 summarises the proposed treatment algorithm, according to the statements.
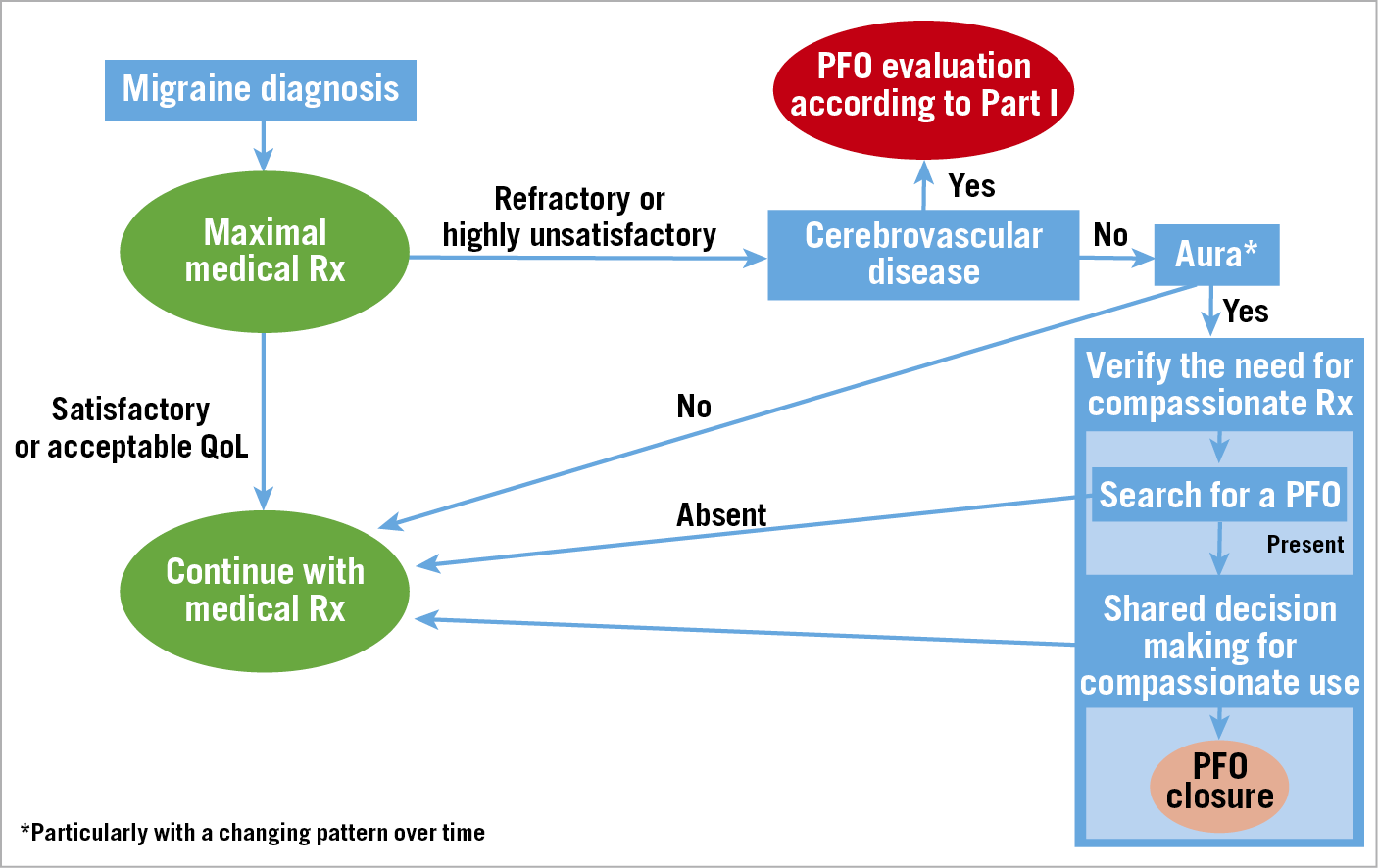
Figure 3. Algorithm for the management of PFO-associated migraine. Rx: therapy
Detailed answers to the PICO question and the detailed characteristics of the considered studies are displayed in Supplementary Table 8 and Supplementary Table 9.
PRACTICAL SUMMARY 2: MIGRAINE
WHAT TO DO
Treat migraine with conventional therapies
Consider PFO closure only in clinical trials or for compassionate use in migraine with aura
WHAT NOT TO DO
Consider PFO closure as part of a routine treatment algorithm
ARTERIAL DEOXYGENATION SYNDROMES
Arterial hypoxaemia is a decrease in the content of oxygen in the blood (SaO2 or SpO2 <90% or PaO2 <60 mmHg), with or without cyanosis. Its main symptoms are exertional and/or resting dyspnoea.
PFO has been associated with several arterial deoxygenation syndromes. Up to 30% of patients with a PFO were discovered to have clinically significant arterial deoxygenation during effort in one study79. To date, only a few studies have been published on this topic. These are summarised in Supplementary Table 10.
CAN PFO BE ASSOCIATED WITH ARTERIAL HYPOXAEMIA? WHAT ARE THE UNDERLYING MECHANISMS?
Several case reports and some experimental and clinical studies have demonstrated that a shunt through a PFO has the potential to cause arterial deoxygenation by mixing venous and arterial blood. In most cases, the PFO shunt only aggravates pre-existing causes of hypoxaemia.
All the causes that elevate pressure in the right heart chambers, such as pulmonary hypertension, can also increase minor shunts. However, several anatomical conditions may also cause a significant shunt, even in the presence of normal mean right atrial pressure (Supplementary Table 10).
DIAGNOSTIC WORKUP
Prior to considering a PFO, an in-depth interdisciplinary diagnostic workup, specific for each clinical syndrome, should be performed to assess the contribution of different potential causes of hypoxaemia, and the pre-test probability of a PFO role in each syndrome should be considered (Supplementary Table 10). The evaluation should be performed and discussed at least by a cardiologist and a pulmonologist.
Every situation in which the baseline condition does not fully explain symptoms and/or the hypoxaemia indicates a need to consider assessing a possible contribution of the PFO.
The diagnostic workup for PFO was published in the first part of this paper1.
In the infrequent case of platypnoea-orthodeoxia syndrome (POS), the most common cause is a PFO80; however, other cardiac and non-cardiac conditions should be ruled out with appropriate tests. A bubble test should be obtained during cardiac imaging with the patient in both a supine and an upright position. The presence of a persistent prominent Eustachian valve may lead to diversion of blood flow from the inferior vena cava towards a PFO. This effect could be exacerbated by atrial deformities and may also lead to a false-negative result during contrast-enhanced transthoracic echocardiography (c-TTE) or contrast transoesophageal echocardiography (c-TOE) via the antecubital vein. A femoral vein contrast injection may be considered in case of high suspicion for POS, prominent Eustachian valve and negative contrast exams.
In obstructive sleep apnoea syndrome (OSAS), it is important to assess the number and severity of episodes of desaturation on therapy to evaluate the possible role of PFO in clinical findings.
Exercise hypoxaemia is significant when there is an SaO2 or SpO2 drop ≥8% from baseline, or to a level <90%.
In all syndromes, a lower-than-expected or absent increase in SaO2 or SpO2 with FiO2 1.0 suggests a significant intracardiac shunt.
Whenever possible, an invasive evaluation of pulmonary pressure to rule out severe pulmonary hypertension and SaO2 measurements (in the left atrium and each pulmonary vein) should be performed to document a step-down in SaO2 while excluding pulmonary abnormalities (pulmonary embolism or intrapulmonary shunts). Moreover, during catheterisation, an occlusion test can demonstrate increased systemic saturation.
IS IT CLINICALLY POSSIBLE TO ESTIMATE THE PROBABILITY OF A CAUSAL RELATIONSHIP BETWEEN A PFO AND HYPOXAEMIA?
Evaluating the role of a PFO in hypoxaemia is difficult and should encompass all the patient’s clinical, imaging and functional data.
In the few available observational studies that have been published, larger and more durably open PFO were the characteristics which correlated more frequently with hypoxaemia in different clinical syndromes.
Invasive measurement of intracardiac arterial oxygen saturation is a key tool for decision making. However, one must consider interference of the catheter in PFO shunting, as well as the difficulty of extrapolating the clinical impact of lab measurements in syndromes in which the opening of a PFO is intermittent.
TREATMENT
Treatment is based on severity of symptoms and the pathogenic role of PFO on shunting. Patients with chronic severe pulmonary hypertension should be excluded from interventional treatment.
No randomised trials have been performed addressing percutaneous closure of PFO in desaturation syndromes.
We performed a meta-analysis of observational before and after closure studies which reported SaO2 or SpO2 for two disparate hypoxaemia syndromes – POS and exertional desaturation. We found a statistically significant increase in SaO2 or SpO2 in both clinical conditions after PFO closure: in exercise desaturation 9.8% (95% CI: 7.1-12.5%) with a severe heterogeneity among studies (I2]: 79%) and in POS 9.6% (95% CI: 5.7-13.5%) also with a severe heterogeneity among studies (I2]: 82%) (Supplementary Figure 5).
In POS due to PFO and OSAS, the evidence for percutaneous closure is based on case reports, case series and small registries. The studies on POS revealed stable relief of symptoms up to five years with improved standing arterial oxygen saturation in all patients who did not have other dominating causes of hypoxaemia81,82,83,84,85. In OSAS, one case-control observational study on 40 patients showed a statistically significant improvement in left ventricular diastolic function, in indices of apnoea and desaturation episodes and a reduction in systemic arterial pressure86.
Only preliminary reports with good results are available for exertional desaturation and high-altitude pulmonary oedema (HAPO), whereas no data are available regarding PFO closure in chronic obstructive pulmonary disease (COPD) patients.
Taken together, these data show that percutaneous closure of PFO has the potential to impact on arterial oxygen saturation and improve symptoms in select patients with an arterial hypoxaemia syndrome. Randomised studies are required to demonstrate effectiveness and safety in these contexts. Position statements are listed in Supplementary Table 11.
PRACTICAL SUMMARY 3: ARTERIAL DEOXYGENATION SYNDROMES
WHAT TO DO
Individually assess and weigh the role of all factors involved in the desaturation syndrome
Whenever possible obtain invasive evidence of the PFO role
Where appropriate, propose PFO closure with shared decision making underscoring the lack of evidence
WHAT NOT TO DO
Routinely close PFO
Close a PFO in the presence of severe chronic pulmonary hypertension
Close a PFO without clear evidence of a crucial role in desaturation
SELECT HIGH-RISK CLINICAL CONDITIONS
PREGNANCY, DELIVERY AND THE PUERPERIUM
Pregnant women are at an increased risk of ischaemic and haemorrhagic stroke and venous thromboembolism compared to non-pregnant women, and PFO-related strokes do happen during pregnancy and the puerperium87,88. However, to date, no large studies have addressed the question of whether PFO is a risk factor for stroke and systemic thrombotic embolisation under such conditions. Specific characteristics of PFO-associated stroke seem to emerge from an analysis of the available reports, but the evidence consists mainly of small case series, so no conclusions can be drawn87. Moreover, no studies have been published testing different preventive approaches for PFO-related stroke. Relevant position statements are listed in Supplementary Table 12.
PREOPERATIVE EVALUATION IN NON-CARDIAC SURGERY
Perioperative stroke, with an incidence ranging from 0.2% to 9.7%, is a serious complication of surgical procedures, with significant consequences in terms of morbidity, duration of hospitalisation and mortality89,90,91.
The incidence of PFO-related stroke during and after surgery and anaesthesia may potentially be increased by haemodynamic changes, hypercoagulability, and the formation of venous thrombosis.
A recent large retrospective study involving 150,198 adult patients who underwent non-cardiac surgery and were extubated after the operation showed a statistically significant increased risk of perioperative ischaemic stroke in patients with a PFO (3.5% vs 0.5%)92. The incidence of stroke in patients with PFO was more significantly increased in otherwise low-risk stroke patients. Moreover, PFO was associated with larger strokes and with more severe neurological deficits and was linked to an increased risk of other systemic embolic complications.
However, there are neither prospective studies addressing these issues, nor RCTs assessing the effectiveness of pharmacotherapy or interventional procedures at decreasing risk.
Relevant position statements are listed in Supplementary Table 12.
NEUROSURGERY IN THE SITTING POSITION
During neurosurgery, after venous incision, a venous air embolism with severe immediate or delayed cardiopulmonary and cerebral complications can potentially occur93,94,95,96,97,98. This occurs more frequently when patients are in a sitting position (up to 50-79% of cases). Adoption of this position has declined considerably99,100,101, also because of other complications102. Notwithstanding this, many surgical teams still place patients in a sitting position as a first choice to approach posterior fossa or dorsally located parietal lesions93,103,104, because of the position’s advantages for surgeons105,106,107,108,109,110,111. In patients with PFO, this results in paradoxical air embolism in up to 14% of the cases112,113,114,115. For this reason, a prone position is usually considered mandatory in safety data116,117. However, paradoxical air embolism can also happen when the patient is prone93.
Diagnostic workup
The diagnostic workup to detect a PFO is described in part I of this document1,2.
Prevention and treatment
Position statements are summarised in Supplementary Table 13.
PERIOPERATIVE MONITORING
During the procedure, patients can be monitored using transoesophageal echocardiography (TOE) and/or transcranial Doppler (TCD). Additionally, end tidal CO2 detects clinically significant venous air emboli118,119. Capnography is a readily available diagnostic tool, with moderate sensitivity and specificity for diagnosing air emboli. An alternative method is to measure expired nitrogen120.
PFO CLOSURE
Since perioperative monitoring can make a timely diagnosis but cannot stop ongoing embolism, preoperative PFO closure has been proposed and presented in extremely limited preliminary reports with good results for the ensuing neurosurgical operation in the sitting position93,121,122.
However, to date, no clinical studies have been published, and questions about the timing of surgery post intervention remain unanswered, especially regarding effective sealing of the defect, the endothelialisation of the device, and the duration of antiplatelet therapy123.
Limitations
This position paper must not be read as a guideline. Indeed, when approaching the statements of this document, one should consider that the included conditions are often uncommon, their pathophysiology still incompletely known, and high-quality data regarding their management are still lacking. The ensuing result is an amount of sparse data with low or very low certainty of evidence. This, of course, has made it impossible to express conclusive focused indications but – since the patients suffering from these syndromes need treatment – has stimulated scientific societies to come together to express shared position statements in order to help approach these conditions rationally according to the available literature.
Conclusion
PFO comes into play in several pathogenic conditions, interacting with other causative processes in disparate dynamic networks. As a result, the heterogeneity of patients is high and evidence, where available, is weak. Therefore, therapeutic solutions often remain empiric, and will probably remain so for a long time due to the low number of patients with similar characteristics, which precludes adequately powered studies. Therefore, beyond the guidelines paradigm which cannot be applied in this context at the moment, this interdisciplinary position paper, based on a comprehensive and strict evaluation of the available data, may be useful for physicians to follow as a broad clinical approach. Nonetheless, based on the published research, we strongly underscore the need for new observational and randomised studies in order to allow the expression of conclusive indications for these poorly focused, and yet clinically relevant, syndromes.
Guest editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Conflict of interest statement
R. Byrne reports personal fees from B. Braun Melsungen AG and from Biotronik, and grants from CeloNova Biosciences, outside the submitted work. B. Dalvi reports other financial activities from Abbott, outside the submitted work. D. Dudek reports grants and personal fees from Abbott, outside the submitted work. D. Hildick-Smith reports personal fees from Abbott, Gore, Occlutech, and Holistick, outside the submitted work. S.E. Kasner reports grants from W.L. Gore, during the conduct of the study, personal fees from Bristol-Myers Squibb and from Boehringer Ingelheim, and grants and personal fees from Medtronic and Bayer, outside the submitted work. J.L. Mas reports personal fees from Abbott, during the conduct of the study. B. Meier reports personal fees from Abbott, outside the submitted work. E.M. Onorato reports personal fees from Occlutech, outside the submitted work. P. Scacciatella reports grants from Abbott Medical and Gore Medical, outside the submitted work. H. Sievert reports reimbursement for clinical trials from 4tech Cardio, Abbott, Ablative Solutions, Ancora Heart, Append Medical, Axon, Bavaria Medizin Technologie GmbH, Bioventrix, Boston Scientific, Carag, Cardiac Dimensions, Cardiac Success, Cardimed, CeloNova, Comed B.V., Contego, CVRx, Dinova, Edwards, Endologix, Endomatic, Hemoteq, Hangzhou Nuomao Medtech, Holistick Medical, K2, Lifetech, Maquet Getinge Group, Medtronic, Mokita, Occlutech, Recor, Renal Guard, Terumo, Trisol, Vascular Dynamics, Vectorious Medtech, Venus, Venock, and Vivasure Medical, outside the submitted work. G. Tarantini reports personal fees from Abbott and Vascular Innovations, during the conduct of the study. J. Thomson reports personal fees from Gore Medical, outside the submitted work. T. Toni reports personal fees from Abbott, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, and Pfizer, outside the submitted work. The chairman of the Task Force and all the other authors declare no conflicts of interest for this work. The Guest Editor has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

