Abstract
Aims: Acute coronary syndrome (ACS) guidelines have been changed, favouring more potent antiplatelet drugs. We aimed to evaluate the safety and efficacy of a ticagrelor- instead of a clopidogrel-based primary dual antiplatelet (DAPT) regimen in ACS patients treated with newer-generation drug-eluting stents (DES).
Methods and results: CHANGE DAPT (clinicaltrials.gov: NCT03197298) assessed 2,062 consecutive real-world ACS patients, treated by percutaneous coronary intervention (PCI), the primary composite endpoint being net adverse clinical and cerebral events (NACCE: all-cause death, any myocardial infarction, stroke or major bleeding). In the clopidogrel (CP; December 2012-April 2014) and ticagrelor periods (TP; May 2014-August 2015), 1,009 and 1,053 patients were treated, respectively. TP patients were somewhat older, underwent fewer transfemoral procedures, and received fewer glycoprotein IIb/IIIa inhibitors. In the TP, the one-year NACCE rate was higher (5.1% vs. 7.8%; HR 1.53 [95% CI: 1.08-2.17]; p=0.02). Assessment of non-inferiority (pre-specified margin: 2.7%) was inconclusive (risk difference: 2.64 [95% CI: 0.52-4.77]; pnon-inferiority=0.48). TP patients had more major bleeding (1.2% vs. 2.7%; p=0.02) while there was no benefit in ischaemic endpoints. Propensity score-adjusted multivariate analysis confirmed higher NACCE (adj. HR 1.75 [95% CI: 1.20-2.55]; p=0.003) and major bleeding risks during TP (adj. HR 2.75 [95% CI: 1.34-5.61]; p=0.01).
Conclusions: In this observational study, the guideline-recommended ticagrelor-based primary DAPT regimen was associated with an increased event risk in consecutive ACS patients treated with newer-generation DES.
Abbreviations
ACS: acute coronary syndrome
ARC: Academic Research Consortium
BMS: bare metal stent
CABG: coronary artery bypass grafting
CP: clopidogrel period
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
MI: myocardial infarction
NACCE: net adverse clinical and cerebral events
PCI: percutaneous coronary intervention
STEMI: ST-segment elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
TP: ticagrelor period
Introduction
In patients with acute coronary syndrome (ACS), many of whom require percutaneous coronary intervention (PCI) with implantation of drug-eluting stents (DES), there is an indication for dual antiplatelet therapy (DAPT). Current international guidelines1,2 recommend the use of more potent P2Y12 inhibitors, such as ticagrelor or prasugrel, instead of the former standard of clopidogrel and aspirin. The pivotal trial that compared ticagrelor versus clopidogrel showed better clinical outcome for ticagrelor in a moderate to high-risk ACS population that comprised 39% of patients who did not undergo PCI during the index hospitalisation3. In addition, more than 60% of trial participants treated by PCI received bare metal stents (BMS), and most patients who received DES had first-generation devices3. Nevertheless, use of this more potent P2Y12 inhibitor was associated with more major bleedings3,4.
Meanwhile, newer-generation DES have become available with thinner stent struts covered by more biocompatible or biodegradable polymer coatings, and improved clinical outcome compared to BMS and first-generation DES5-7. In clinical practice, most ACS patients are treated with newer-generation DES that showed favourable results with clopidogrel-based DAPT8,9. In the present study, we evaluate the impact of the guideline-recommended change in primary DAPT regimen to ticagrelor on one-year outcome in consecutive ACS patients treated with newer-generation DES at a high-volume PCI centre.
Methods
STUDY POPULATION AND DESIGN
At the tertiary PCI centre Thoraxcentrum Twente in the Netherlands we performed an investigator-initiated, prospective observational study that assessed one-year outcome in 2,062 consecutive ACS patients, treated with newer-generation DES (CHANGE DAPT, NCT03197298). Patients were included between 21 December 2012 and 25 August 2015. On 1 May 2014, we followed international guidelines to replace clopidogrel- by ticagrelor-based primary DAPT. Group 1 patients were included before (clopidogrel period [CP]) and group 2 patients after this date (ticagrelor period [TP]). ACS patients ≥18 years, treated with newer-generation DES, were included. Exclusion criteria were known pregnancy, life expectancy <1 year (i.e., no cardiogenic shock or post-resuscitation), planned elective surgery requiring DAPT interruption after <6 months, and known intolerance to DES components. As guidelines recommend clopidogrel use in patients on oral anticoagulation2, patients with oral anticoagulation at baseline were not included.
This observational study complied with the Declaration of Helsinki. According to Dutch law, and as approved by the Medical Ethics Committee Twente, written informed consent was not required.
DEFINITIONS OF CLINICAL ENDPOINTS
The primary endpoint net adverse clinical and cerebral events (NACCE) is a composite of all-cause death, any myocardial infarction (MI), stroke or major bleeding. Definitions of MI are according to the Academic Research Consortium (ARC)10,11. Strokes were a focal loss of neurologic function by an ischaemic or haemorrhagic event, with residual symptoms ≥24 hours or leading to death. Bleeding ARC (BARC) and Thrombolysis In Myocardial Infarction (TIMI) bleeding criteria were used12,13. Major bleeding was any BARC class 3 or 5 bleeding and/or all TIMI major bleedings (i.e., coronary artery bypass grafting [CABG] and non-CABG-related).
Secondary endpoints were individual components of the primary composite endpoint, any clinically indicated revascularisation by PCI or CABG, definite or probable stent thrombosis according to ARC10, the composite of cardiac death, MI or stroke.
CORONARY INTERVENTION AND MEDICAL THERAPY
Interventional procedures were performed according to local clinical protocols. Unfractionated heparin was administered directly before PCI. Lesion preparation, stent post-dilation and use of glycoprotein IIb/IIIa inhibitors (generally limited to a single bolus of abciximab) were left to the operator’s discretion. If patients were not on antiplatelet therapy, loading doses of aspirin (≥300 mg) and clopidogrel (600 mg) or ticagrelor (180 mg) were administered before PCI. Timing of drug loading did not change during the study. Maintenance doses were 80-100 mg aspirin and 75 mg clopidogrel daily, and/or 90 mg ticagrelor twice daily. DAPT of patients referred from other hospitals generally remained unchanged. DAPT was generally prescribed for 12 months with the use of statins, beta-blockers, RAS inhibitors, and proton pump inhibitors as appropriate according to guidelines and the physician’s judgement. If patients required oral anticoagulation during follow-up, ticagrelor was replaced by clopidogrel, and aspirin was generally stopped one month after PCI.
CLINICAL FOLLOW-UP AND EVENT ASSESSMENT
Information on clinical follow-up was obtained at visits to outpatient clinics and/or by mail or telephone. The contract research organisation Cardio Research Enschede (Enschede, the Netherlands) performed the study. During both DAPT periods, similar numbers of patients participated in a DES trial14 with independent data monitoring. Clinical events were adjudicated by a committee (three members of the research team), and strokes were assessed by an experienced neurologist.
STATISTICAL ANALYSIS
Data were reported as frequencies and percentages or mean±standard deviation. Differences in dichotomous and categorical variables were assessed by chi-square test, while continuous variables were assessed with the Student’s t-test. Kaplan-Meier analysis was used to calculate the time to clinical endpoints; the log-rank test was applied for between-group comparisons. Hazard ratios were computed using Cox proportional hazards regression analyses. For adjustment of potential confounders, a propensity score analysis was performed. Propensity scores were estimated using multiple logistic regression analysis. All baseline and procedural variables were used to calculate the propensity score for treatment in the TP; a multivariate Cox regression model was used to adjust for the propensity score.
The primary analyses compared the two treatment periods, CP versus TP. To assess non-inferiority, a NACCE rate of 6.5% was assumed in both periods (i.e., CP and TP), based on the data from the OPTIMIZE, TWENTE and DUTCH PEERS trials8,9,15. With a one-sided alpha level of 0.05 and at least 80% power, a sample size of 1,031 patients in each group was required to demonstrate non-inferiority with a margin of 2.7%, based on the OPTIMIZE trial15. Non-inferiority would be achieved if the upper limit of the one-sided 95% confidence interval of the absolute risk difference was less than the non-inferiority margin.
Additional sensitivity analyses were performed, in which patients actually treated with clopidogrel during the CP were compared to patients actually treated with ticagrelor during the TP. Treatment by either clopidogrel or ticagrelor was assessed at discharge or, if a NACCE occurred before discharge, at the time of that in-hospital event.
Except for the non-inferiority analysis, p-values and confidence intervals were two-sided; p-values <0.05 were considered significant. Sample size calculation was performed with PASS (NCSS, Kaysville, UT, USA), and data analysis with SPSS, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
STUDY POPULATION
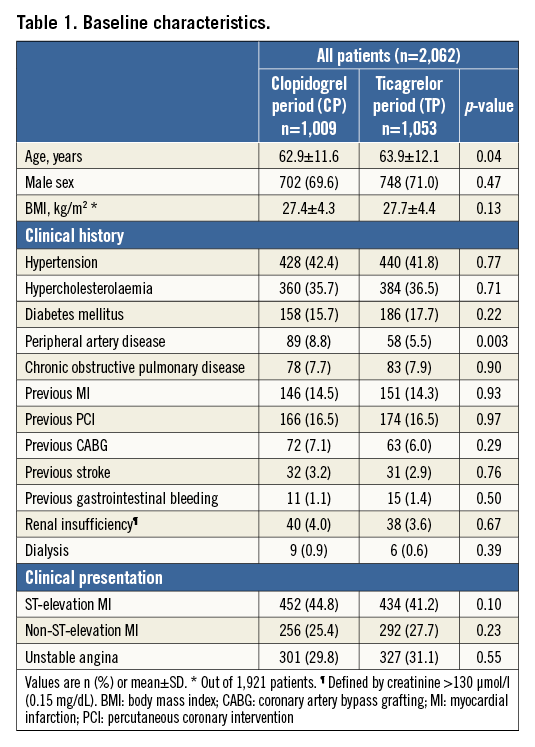
Of all 2,062 patients enrolled, 1,009 (48.9%) were treated during the CP versus 1,053 (51.1%) during the TP (Figure 1). Most patient demographic and clinical characteristics (Table 1) were similar for both periods, including the proportion of STEMI (44.8% vs. 41.2%). TP patients were somewhat older (62.9±11.6 vs. 63.9±12.1 years, p=0.04) and less often diagnosed with peripheral artery disease (8.8% vs. 5.5%, p=0.003).
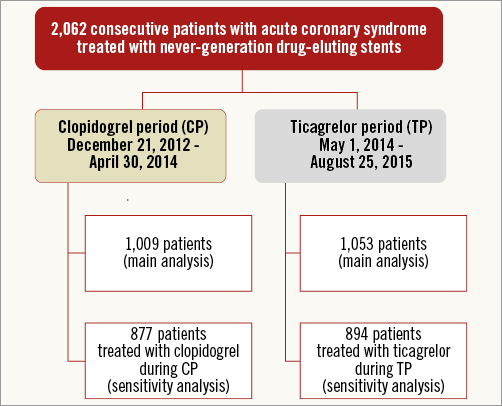
Figure 1. Flow chart. Exact number of patients not fulfilling inclusion criteria not available.
INTERVENTIONAL PROCEDURE
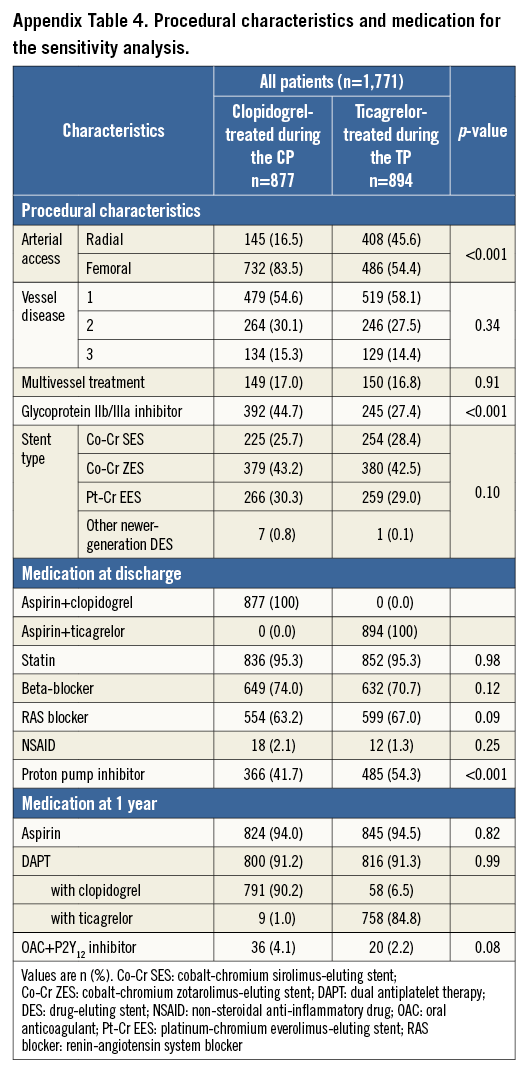
Details of PCI and medication are shown in Table 2. Multivessel treatment and stent type did not differ between periods, but transradial access was more common during the TP (17.7% vs. 44.6%, p<0.001).
MEDICATION
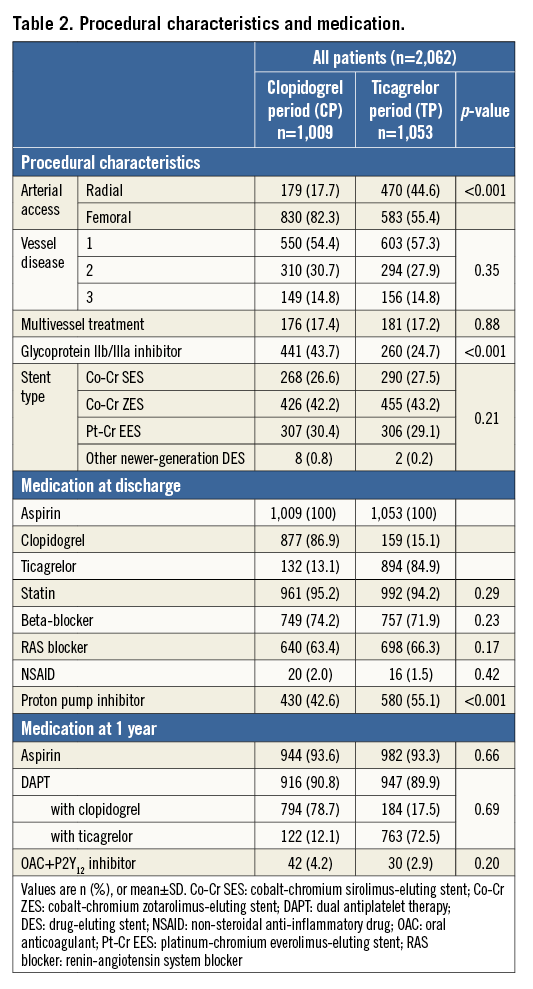
The use of glycoprotein IIb/IIIa inhibitors decreased from CP to TP (43.7% vs. 24.7%, p<0.001) (Table 2). More TP patients were treated with a proton pump inhibitor at discharge (42.6% vs. 55.1%, p<0.001).
During the CP, 877/1,009 (86.9%) patients were treated with clopidogrel at discharge, while 132/1,009 (13.1%) were on ticagrelor. At one-year follow-up, 916/1,009 (90.8%) CP patients were still on DAPT: 78.7% used clopidogrel and 12.1% ticagrelor; a combination of oral anticoagulant with P2Y12 inhibitor was prescribed in 42/1,009 (4.2%).
During the TP, 894/1,053 (84.9%) patients were on ticagrelor and 159/1,053 (15.1%) on clopidogrel. At one-year follow-up, 947/1,053 (89.9%) patients were still on DAPT: 17.5% used clopidogrel and 72.5% ticagrelor; an oral anticoagulant plus P2Y12 inhibitor was prescribed in 30/1,053 (2.9%) patients.
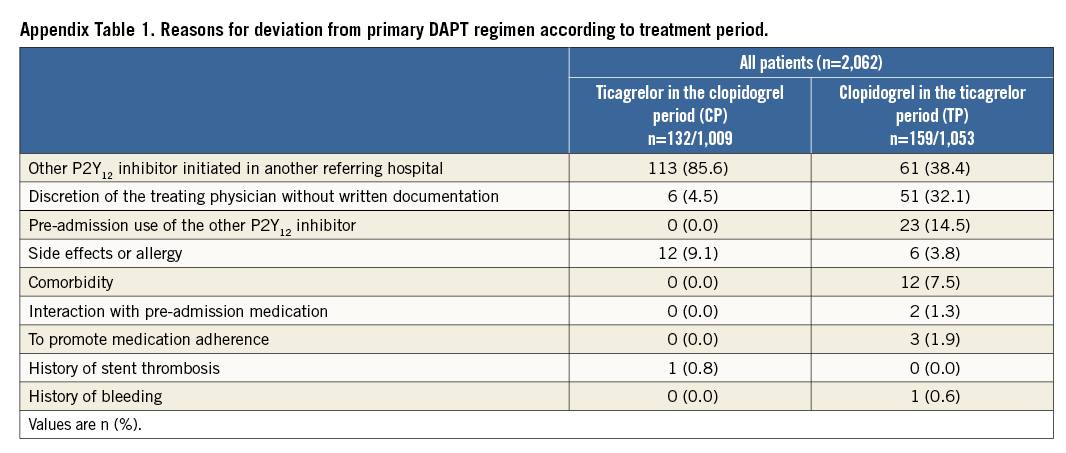
Reasons for deviation from the primary DAPT regimen at baseline are presented in Supplementary Table 1. The main reason for ticagrelor use during the CP was that DAPT was initiated in referring hospitals. During the TP, clopidogrel was primarily used because of DAPT initiation in referring hospitals or at the discretion of treating physicians (without written motivation).
CLINICAL OUTCOME
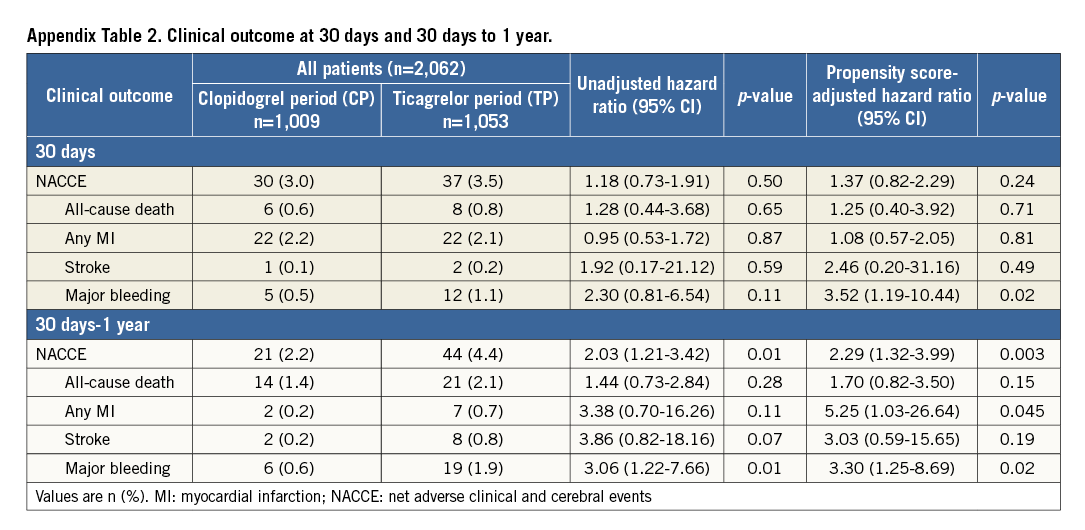
One-year clinical follow-up was available in 2,048/2,062 (99.3%) patients (seven lost in each period). Data of 30-day, and 30-day to one-year outcomes are presented in Supplementary Table 2. Table 3 reports one-year outcomes of various clinical endpoints during the CP and TP. The composite primary endpoint NACCE occurred in 51/1,009 (5.1%) CP patients versus 81/1,053 (7.8%) TP patients (HR 1.53 [95% CI: 1.08-2.17], p=0.02). Assessment of non-inferiority of TP versus CP resulted in inconclusive findings (risk difference 2.64 [95% CI: 0.52-4.77], pnon-inferiority=0.48). The higher NACCE rate in TP patients was largely caused by a difference in major bleeding (1.2% vs. 2.7%, p=0.02). There was no significant difference in the individual endpoints all-cause death, MI, and stroke; the same applied to the composite of cardiac death, MI or stroke (3.7% vs. 4.7%, p=0.27). Figure 2 and Figure 3 display the time-to-event curves for the primary endpoint NACCE and its individual components. During the CP and TP, rates of definite (0.3% vs. 0.6%; p=0.35) and definite or probable stent thrombosis (0.6% vs. 0.8%; p=0.65) were low.
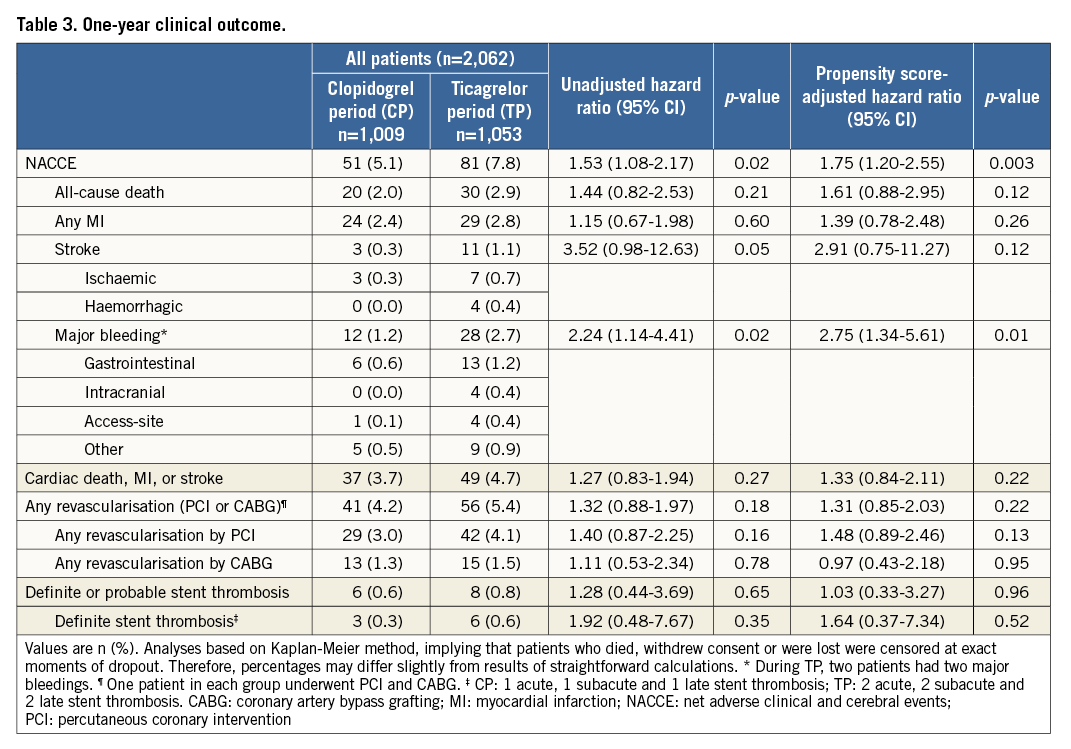
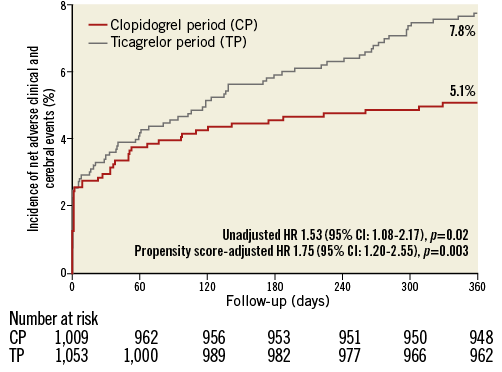
Figure 2. One-year Kaplan-Meier cumulative incidence curves for the primary endpoint NACCE. CI: confidence interval; HR: hazard ratio; NACCE: net adverse clinical and cerebral events
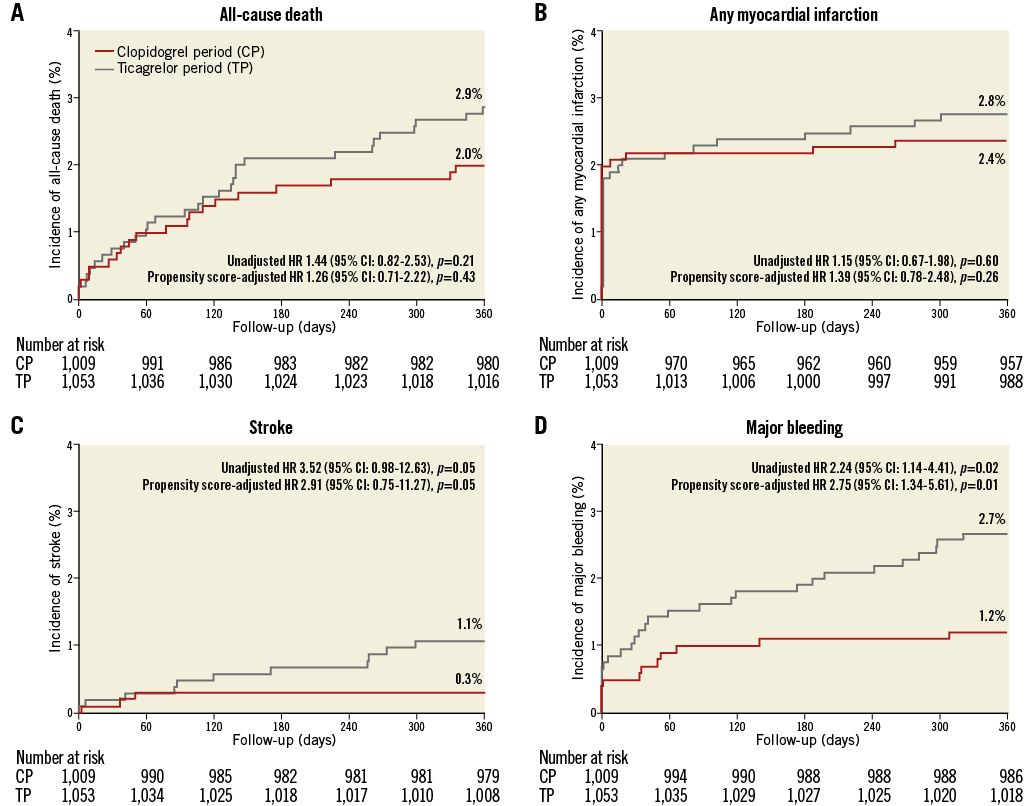
Figure 3. One-year Kaplan-Meier cumulative incidence curves for the individual components of the primary endpoint. A) All-cause death. B) Any myocardial infarction. C) Stroke. D) Major bleeding. CI: confidence interval; HR: hazard ratio
Propensity score-adjusted multivariate analysis confirmed significantly higher rates of NACCE (adjusted HR 1.75, p=0.003) and major bleeding (adjusted HR 2.75, p=0.01) during the TP (Table 3).
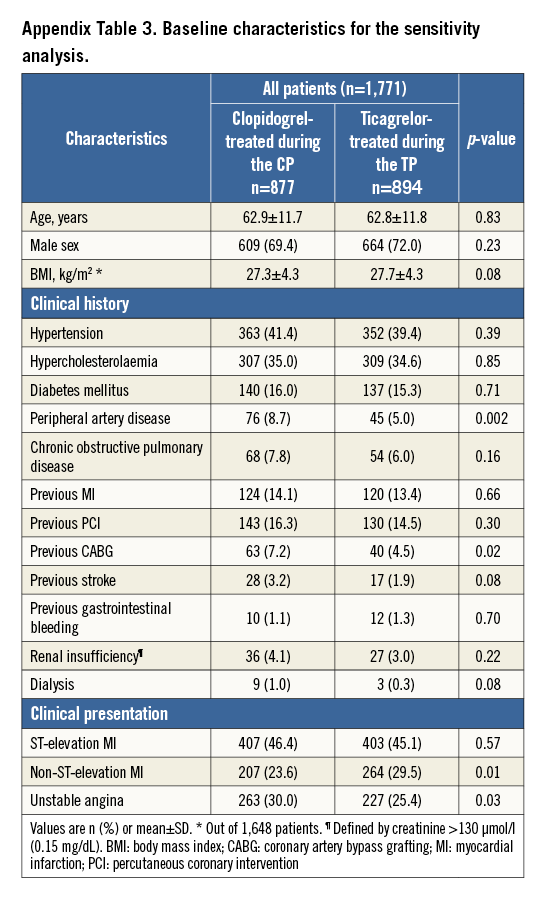
SENSITIVITY ANALYSIS
Supplementary Table 3 and Supplementary Table 4 present the baseline characteristics of the study population of the sensitivity analysis; clinical outcomes are presented in Table 4. In clopidogrel-treated patients during the CP versus ticagrelor-treated patients during the TP, the NACCE rate was 5.2% vs. 7.0% (HR 1.33 [95% CI: 0.92-1.99], p=0.12); the risk difference was 1.80 [95% CI: -0.43-4.06], pnon-inferiority=0.21). There was a significantly higher incidence of major bleeding in ticagrelor-treated patients during the TP (1.1% vs. 2.7%, HR 2.36 [95% CI: 1.13-4.93], p=0.02).
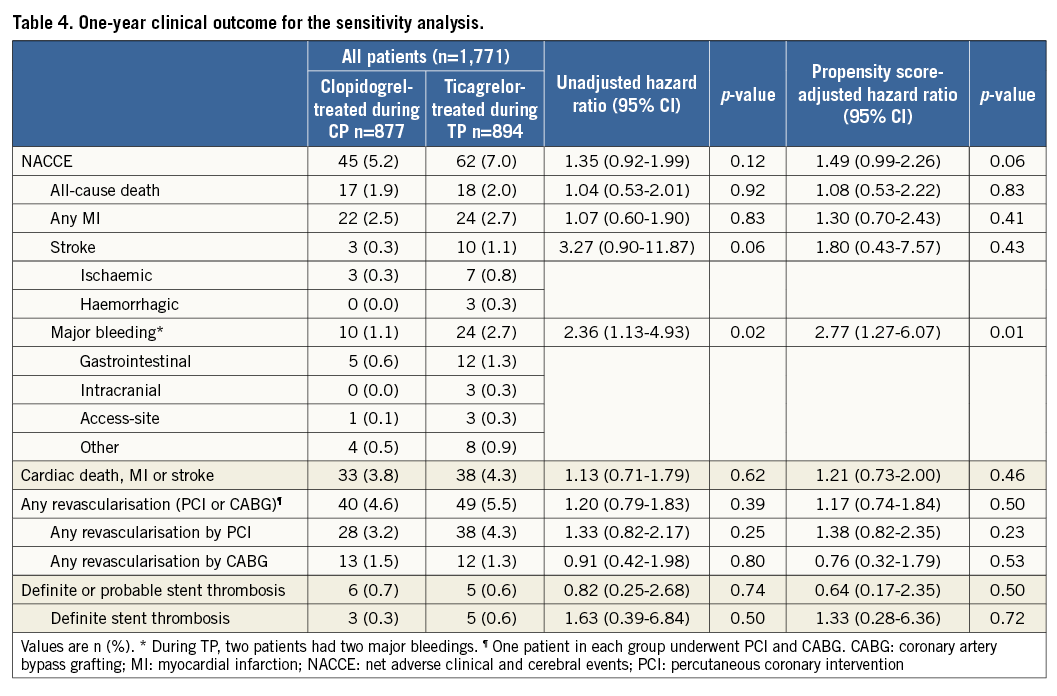
After propensity score-adjusted multivariate analysis, this difference in major bleeding was still significant (adjusted HR 2.77 [95% CI: 1.27-6.07], p=0.01), while for NACCE the numeric difference did not reach statistical significance (adjusted HR 1.49 [95% CI: 0.99-2.26], p=0.06).
Discussion
Patients treated during the TP had significantly higher rates of the primary endpoint NACCE as compared to CP patients, and non-inferiority assessment of the ticagrelor-based primary DAPT regimen (as compared to clopidogrel-based DAPT) showed inconclusive results. During the TP there were significantly more major bleedings. The rates of all-cause death, MI and stroke showed no statistically significant difference between treatment periods. Propensity score-adjusted multivariate analyses demonstrated that treatment during the TP was an independent predictor of NACCE and major bleedings. Patients treated with ticagrelor during the TP had a significantly higher risk of major bleedings than patients treated with clopidogrel during the CP, while there was no difference in ischaemic outcomes.
International guidelines recommend the use of ticagrelor over clopidogrel in all ACS patients merely based on the PLATO (PLATelet Inhibition and Patient Outcomes) trial, in which ticagrelor-treated STEMI and other moderate to high-risk ACS patients had significantly lower rates of vascular death and MI than clopidogrel-treated patients3. In a recent meta-analysis, STEMI patients undergoing PCI were found to have a lower mortality and fewer major adverse cardiac events if ticagrelor-based DAPT was used; however, this was mainly driven by data from PLATO with marginal additional input from a few small-sized studies with short duration (1-3 months) of DAPT and follow-up16. In contrast to PLATO, the randomised PHILO trial found non-significantly higher rates of major adverse cardiovascular events and major bleeding in Asian ACS patients treated with ticagrelor versus clopidogrel17.
Randomised controlled trials are considered the gold standard of clinical research, but they only have limited external validity, as trial participants frequently differ from patients in a real-world setting. For instance, participants in randomised trials with unregistered drugs may represent a special category of patients, who are likely to have a better medication adherence than patients in “real-world” registries3,18. Therefore, positive findings of randomised controlled trials should be confirmed in broader patient populations, as are examined in large real-world registries, which provide complementary information. In the 45,073 ACS patients of the real-world SWEDEHEART registry, treated with or without PCI, comparable results to PLATO were found with a lower mortality in patients on ticagrelor19; however, in SWEDEHEART, ticagrelor was preferentially used in patients at low risk of bleeding and death (indicated by lower CRUSADE and GRACE scores)20, and patients on ticagrelor were significantly more often assessed by coronary angiography and treated by PCI19,20. Furthermore, in the GRAPE registry, which examined ACS patients treated by PCI, ticagrelor- and clopidogrel-treated patients showed similar major adverse cardiac event rates21. These studies suggest that, so far, no equivocal decision can be made on whether ticagrelor is superior in real-world clinical practice.
The main findings of CHANGE DAPT corroborate results of the recent TOPIC trial, which showed no differences in ischaemic outcomes at one-year follow-up between more potent P2Y12 inhibitor- versus clopidogrel-treated ACS patients, and a net clinical benefit for switching to clopidogrel-based DAPT22. In the TOPIC study, ACS patients treated by PCI were randomised after one month of DAPT with more potent P2Y12 inhibitors, to continued treatment with the potent P2Y12 inhibitor until 12 months, or to switching to clopidogrel. The main outcome consisted of a net clinical benefit for the switched group, primarily driven by a significantly higher bleeding risk in patients with a continued potent P2Y12 inhibitor22. Furthermore, a preliminary analysis of SCAAR registry data in 12,168 patients from Västra Götaland County, treated for ACS with PCI, also found no mortality benefit for ticagrelor use, but major bleedings were not assessed (Omerovic E. Ticagrelor is not superior to clopidogrel in patients with acute coronary syndrome: a report from SCAAR. Presented at: EuroPCR 2017, Paris, France, 18 May 2017. Available at https://www.pcronline.com/Cases-resources-images/Resources/Course-videos-slides/2017/Antiplatelet-regimen-after-PCI-an-ongoing-debate [last visited July 24, 2017]).
The higher rates of major bleedings for ticagrelor-treated patients in the present study are in line with other studies3,19,21, but were not associated with an increased mortality, as observed by others23,24. In CHANGE DAPT, ticagrelor-treated patients had a higher bleeding risk despite more transradial procedures and less glycoprotein IIb/IIIa inhibitor use – two factors known to reduce periprocedural bleedings. Furthermore, during the TP, proton pump inhibitors were more frequently prescribed to prevent gastrointestinal bleedings – the most common type of major bleedings.
Stroke rates during the CP were comparable to OPTIMIZE15 and during the TP similar to PLATO3. During both DAPT periods, revascularisation and stent thrombosis rates were generally low and did not differ significantly between groups. The low event rates could well be attributed to experienced operators, liberal use of stent post-dilation and use of newer-generation DES only.
Limitations
Inherent to the study design, patients were not randomised. During both DAPT periods, certain patients were treated with the other P2Y12 inhibitor. Although there were only a few differences in baseline characteristics, and propensity score-adjusted multivariate analyses were performed to adjust for potential confounders, residual confounding cannot be excluded. In addition, our study was not powered for detecting differences in clinical outcome with low event rates such as death, stroke, and stent thrombosis.
Despite >99% follow-up in our study, which limits the probability of event underreporting, ischaemic and bleeding events were lower than in previous randomised DAPT trials3. Nevertheless, this is in line with the low event rates of our randomised stent trials, in which the vast majority of PCI procedures were performed by senior operators with large individual experience9,14. Moreover, the NACCE rate was comparable to OPTIMIZE15 on which the power calculation of CHANGE DAPT was based. Other contemporary registries in real-world STEMI patients also showed lower event rates than in phase III studies25. These differences may be partially explained by ascertainment bias and by dissimilarities in study design, endpoint definition, and patient population. The PLATO trial, for instance, comprised ACS patients treated by optimal medical therapy, PCI or bypass surgery; of all the patients treated by PCI, more than 60% received BMS only3. As BMS use increases major adverse cardiac event risk6,7,26, the exclusive use of newer-generation DES in our study could have lowered the MI and revascularisation rates; the latter may have contributed to the lower bleeding rates observed. CHANGE DAPT findings should not be generalised to ACS patients who undergo non-PCI-based treatment.
Conclusions
In this observational study, the guideline-recommended primary DAPT regimen was associated with an increased adverse event risk in consecutive ACS patients, treated by PCI with newer-generation DES. The difference in event risk was primarily driven by the rate of major bleeding. Assessment of non-inferiority of ticagrelor-based DAPT for the primary endpoint NACCE was inconclusive.
| Impact on daily practice Based on international guidelines, ticagrelor has largely replaced clopidogrel as a component of DAPT in ACS patients. CHANGE DAPT confirms that treatment with ticagrelor is associated with more major bleedings. This increased bleeding risk should be balanced against benefits in reducing ischaemic events, as previously found in broad populations of ACS patients. Nevertheless, in ACS patients treated by PCI with newer-generation DES, the benefits of ticagrelor in reducing ischaemic events have not yet been demonstrated. In CHANGE DAPT these benefits were not observed, but we cannot exclude that certain subgroups of PCI patients, treated with contemporary DES, may benefit from ticagrelor use. |
Funding
The CHANGE DAPT study was performed without external funding. The research department of Thoraxcentrum Twente has received research grants provided by AstraZeneca, Biotronik, Boston Scientific, and Medtronic. Data acquisition was partially supported by an unrestricted research grant from AstraZeneca.
Conflict of interest statement
P. Zocca has received a lecture fee from AstraZeneca. The other authors have no conflicts of interest to declare.
Supplementary data