Abstract
Background: In the TWILIGHT trial, ticagrelor monotherapy after a short course of dual antiplatelet therapy (DAPT) was shown to be a safe bleeding avoidance strategy in high-risk patients undergoing percutaneous coronary intervention (PCI) with drug-eluting stents (DES).
Aims: The aim of this study was to evaluate the effects of ticagrelor monotherapy after three-month DAPT in patients undergoing PCI, according to DES type.
Methods: In the current sub-analysis from TWILIGHT, patients were stratified into three groups based on DES type: durable polymer everolimus-eluting stents (DP-EES), durable polymer zotarolimus-eluting stents (DP-ZES), and biodegradable polymer DES (BP-DES). Bleeding and ischaemic outcomes were assessed at one year after randomisation.
Results: Out of 5,769 patients, 3,014 (52.2%) had DP-EES, 1,350 (23.4%) had DP-ZES and 1,405 (24.4%) had BP-DES. Compared with ticagrelor plus aspirin, ticagrelor monotherapy had significantly lower BARC type 2, 3 or 5 bleeding compared with DAPT; DP-EES (3.8% vs 6.7%; HR 0.56, 95% CI: 0.41-0.78), DP-ZES (4.6% vs 6.9%; HR 0.66, 95% CI: 0.42-1.04) and BP-DES (4.2% vs 7.9%; HR 0.52, 95% CI: 0.33-0.81; pinteraction=0.76). Ticagrelor monotherapy resulted in similar rates of death, MI, or stroke: DP-EES (4.2% vs 4.3%; HR 0.97; 95% CI: 0.68-1.37); DP-ZES (4.1% vs 3.1%; HR 1.32; 95% CI: 0.75-2.33); BP-DES (3.9% vs 4.2%; HR 0.92; 95% CI: 0.54-1.55; pinteraction=0.60). In both unadjusted and covariate-adjusted analyses, DES type was not associated with any differences in ischaemic or bleeding complications.
Conclusions: As compared with ticagrelor plus aspirin, ticagrelor monotherapy after a short DAPT duration lowered bleeding complications without increasing the ischaemic risk, irrespective of DES type. We observed no significant differences among DES types.
Introduction
Dual antiplatelet therapy (DAPT) with aspirin plus a P2Y12-receptor inhibitor constitutes the standard of care following percutaneous coronary intervention (PCI) with drug-eluting stents (DES) to prevent coronary thrombotic events1. First-generation DES, while more effective than bare metal stents at reducing rates of restenosis, were limited by late and very late thrombosis2. Iterations in DES technologies with refinements in stent design, drug, polymer and alloy as well as more potent P2Y12-receptor inhibitors further improved the safety of PCI by reducing the incidence of early and late thrombotic complications34. Prolonged DAPT, while effective in reducing long-term ischaemic events, results in significantly higher rates of major bleeding complications which are in turn associated with increased risk of morbidity and mortality56789. These observations led to a series of studies evaluating the safety and efficacy of abbreviated DAPT duration consisting of early P2Y12-receptor inhibitor withdrawal following PCI with DES10.
An emerging strategy of early aspirin withdrawal (i.e., 1-3 months post-PCI) with continuation of P2Y12-receptor inhibitor has recently been demonstrated to reduce bleeding risk while preserving ischaemic protection11. Monotherapy with the potent P2Y12-receptor inhibitor ticagrelor following three months of DAPT resulted in a lower incidence of clinically relevant bleeding without increasing the risk of ischaemic events, compared to continuing DAPT up to 15 months post-PCI with DES11. Patients undergoing PCI with different stent types may have variable ischaemic/bleeding risk profiles (i.e., due to large differences in strut thickness, polymer type, eluting drug, etc.) and thus may respond differently to this novel strategy. A prior sub-analysis from the TWILIGHT trial showed the safety and efficacy of ticagrelor monotherapy in patients receiving the SYNERGY biodegradable polymer drug-eluting stents (BP-DES)12. A broader evaluation across various platforms of durable polymer drug-eluting stents (DP-DES) and BP-DES has not been performed. We therefore performed a post hoc analysis of the Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention (TWILIGHT) trial evaluating the safety and efficacy of a regimen of ticagrelor monotherapy versus ticagrelor plus aspirin in patients who initially completed three months of DAPT after PCI with different types of new-generation DES.
Methods
Study design
TWILIGHT was an international, multicentre, randomised, placebo-controlled trial conducted in 187 sites across 11 countries, as previously described1113. The Icahn School of Medicine at Mount Sinai designed and sponsored the trial, which was supported by an investigator-initiated grant from AstraZeneca. National regulatory agencies and institutional review boards or ethics committees of participating centres approved the trial protocol. An independent data and safety monitoring board provided external oversight to ensure the safety of the trial participants.
Study population
Patients who underwent successful PCI with at least one locally approved DES and whom the treating clinician intended to discharge on a regimen of ticagrelor plus aspirin were eligible to participate. Patients also had to have at least one additional clinical feature and one angiographic feature associated with a high risk of ischaemic or bleeding events13. For the present pre-specified analysis, only durable polymer everolimus-eluting stents (DP-EES), durable polymer zotarolimus-eluting stents (DP-ZES) and BP-DES were included (Supplementary Figure 1). Patients who underwent PCI with more than one stent type or with a bare metal stent implanted were excluded. A full list of the commercially approved DES types included in the analysis is provided in Supplementary Table 1. The clinical criteria for high risk were: age ≥65 years, female sex, troponin-positive acute coronary syndrome (ACS), established vascular disease, diabetes mellitus that was being treated with medication (including both oral and parenteral medications), and chronic kidney disease. Angiographic criteria included multivessel coronary artery disease (CAD), a total stent length >30 mm, a thrombotic target lesion, a bifurcation lesion treated with two stents, an obstructive left main or proximal left anterior descending lesion, and a calcified target lesion treated with atherectomy. Key exclusion criteria included presentation with ST-segment elevation myocardial infarction, cardiogenic shock, ongoing long-term treatment with oral anticoagulants, or contraindication to aspirin or ticagrelor.
Study procedures
All enrolled patients received open-label ticagrelor (90 mg twice daily) and enteric-coated aspirin (81 to 100 mg daily) after the index PCI. At the three-month follow-up visit, patients who remained adherent and had not sustained a major bleeding event (defined as a Bleeding Academic Research Consortium [BARC] type 3b or 5 bleed) or a major ischaemic event (stroke, myocardial infarction, or coronary revascularisation) were eligible for randomisation to either aspirin (81 to 100 mg daily) or matching placebo with continuation of open-label ticagrelor (90 mg twice daily) for an additional 12 months. The choice of prolonged potent DAPT in the control group was justified by the heightened ischaemic risk, as reflected by the procedural/angiographic inclusion criteria of the study population1113. Follow-up was performed by telephone at one month after randomisation and in person at six and 12 months after randomisation. Adherence was assessed with manual pill counts, and non-adherence was classified systematically, as described previously14. After 12 months of protocol-mandated therapy, patients were switched to a standard-of-care antiplatelet regimen at the discretion of their treating physician, followed by a final telephone follow-up three months later.
Endpoints
The primary endpoint of the study was BARC type 2, 3 or 5 bleeding15 between randomisation and the one-year follow-up (i.e., 15 months after the index procedure). The key secondary endpoint was major adverse cardiac and cerebrovascular events (MACCE), defined as a composite of death from any cause, non-fatal myocardial infarction (MI) or non-fatal stroke. Secondary bleeding endpoints included BARC type 3 or 5 bleeding15; Thrombolysis in Myocardial Infarction (TIMI) major or minor bleeding16; Global Use of Strategies to open Occluded Arteries (GUSTO) moderate, severe, or life-threatening bleeding17; or major bleeding as defined by the International Society on Thrombosis or Haemostasis (ISTH)18. Other secondary endpoints included death from cardiovascular causes, MI, ischaemic stroke, and definite or probable stent thrombosis (ST). MI was defined according to the third universal definition19, and revascularisation and ST were classified according to the Academic Research Consortium20. The definitions of study endpoints are listed in Supplementary Table 2. All clinical events were adjudicated by an external independent committee, the members of which were unaware of the treatment group assignments.
Statistical analysis
Analyses were performed in the intention-to-treat population for bleeding endpoints and in the per-protocol population for ischaemic endpoints. Baseline characteristics were compared using chi-square or Student’s t-test for categorical or continuous variables, respectively. The cumulative incidence of the primary and secondary endpoints was estimated by the Kaplan–Meier method. Hazard ratios (HR) and 95% confidence intervals (CI) were generated using adjusted Cox proportional hazards models for DES-type comparisons. Clinically relevant variables were included in the adjustment model: body mass index (kg/m2), hypercholesterolaemia, peripheral arterial disease, previous PCI or coronary artery bypass graft surgery, multivessel CAD, indication for PCI (ACS versus stable CAD), total occlusion of target vessel, and total stent length. The consistency of the treatment effect of ticagrelor monotherapy versus ticagrelor plus aspirin between the different stent types (DP-EES, DP-ZES and BP-DES) was evaluated with formal interaction testing. All analyses were performed using Stata version 16.0 (College Station, TX, USA). A p-value <0.05 indicates statistical significance.
Results
A total of 9,006 patients were initially enrolled following PCI, of which 7,119 were randomly assigned three months later to receive ticagrelor plus placebo or ticagrelor plus aspirin. Of these 7,119 patients, 5,769 (81.0%) were included in this analysis. Of these, 3,014 (52.2%) received a DP-EES, 1,350 (23.4%) received a DP-ZES and 1,405 (24.4%) received a BP-DES. The study flow diagram is reported in Supplementary Figure 1. Baseline clinical and procedural characteristics for patients according to type of new-generation DES are reported in Table 1; similarly, baseline characteristics according to treatment arm within each stent type group are reported in Supplementary Table 3. Patients who underwent PCI with a BP-DES had fewer comorbidities and were more likely to present with an ACS. Overall outcomes according to the three types of DES are reported in Figure 1-Figure 3 and Supplementary Table 4; in both univariate analysis and multivariable analyses, DES type was not associated with an increased risk of MACCE, target lesion failure (TLF) or major bleeding complications. One-year rates of stent thrombosis were <1% across all DES platforms.
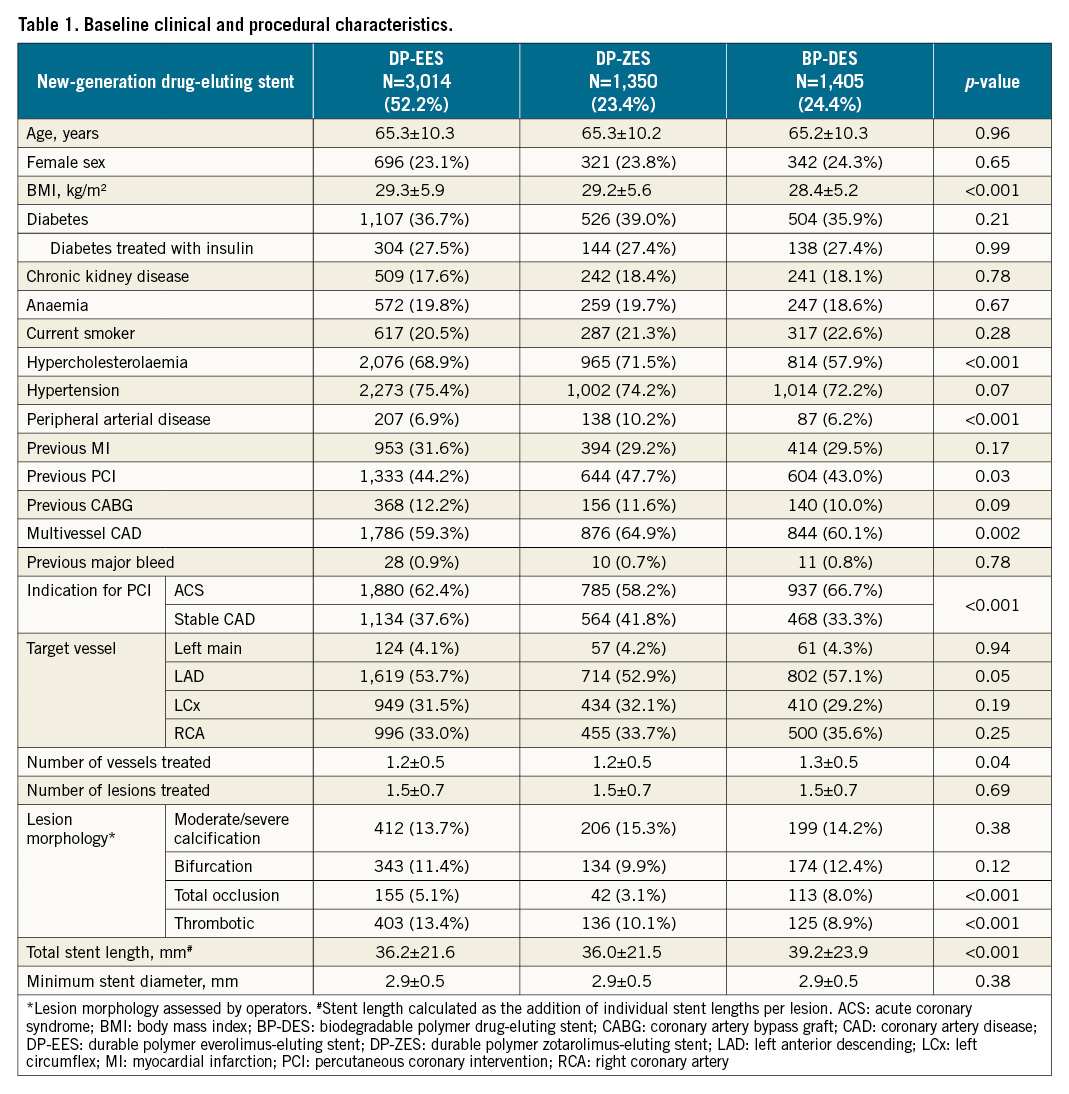
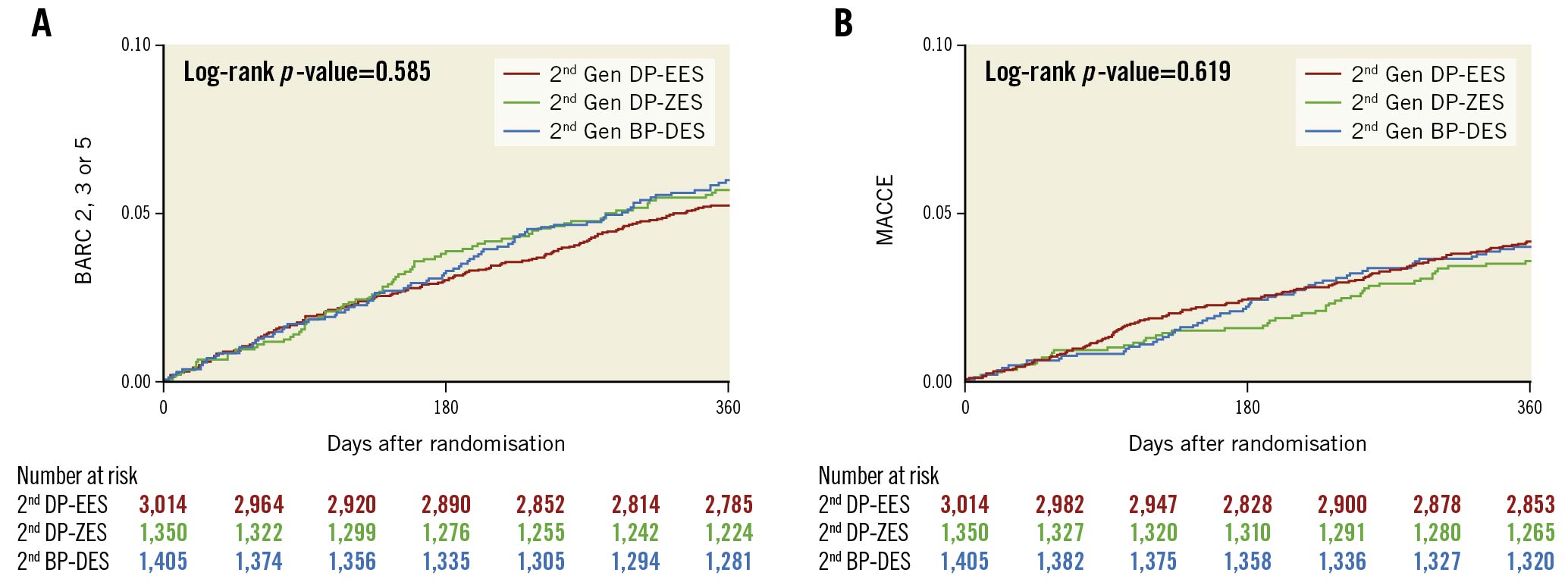
Figure 1. Rates of (A) BARC 2, 3 or 5 bleeding and (B) MACCE among the three DES types evaluated. Kaplan–Meier estimates for BARC 2, 3 or 5 bleeding and target lesion failure at 12 months after randomisation (intention-to-treat population) by drug-eluting stent type in patients who underwent percutaneous coronary intervention. BARC: Bleeding Academic Research Consortium; BP-DES: biodegradable polymer drug-eluting stent; DP-EES: durable polymer everolimus-eluting stent; DP-ZES: durable polymer zotarolimus-eluting stent; Gen: generation; MACCE: major adverse cardiac and cerebral events (all-cause death, myocardial infarction, or stroke)
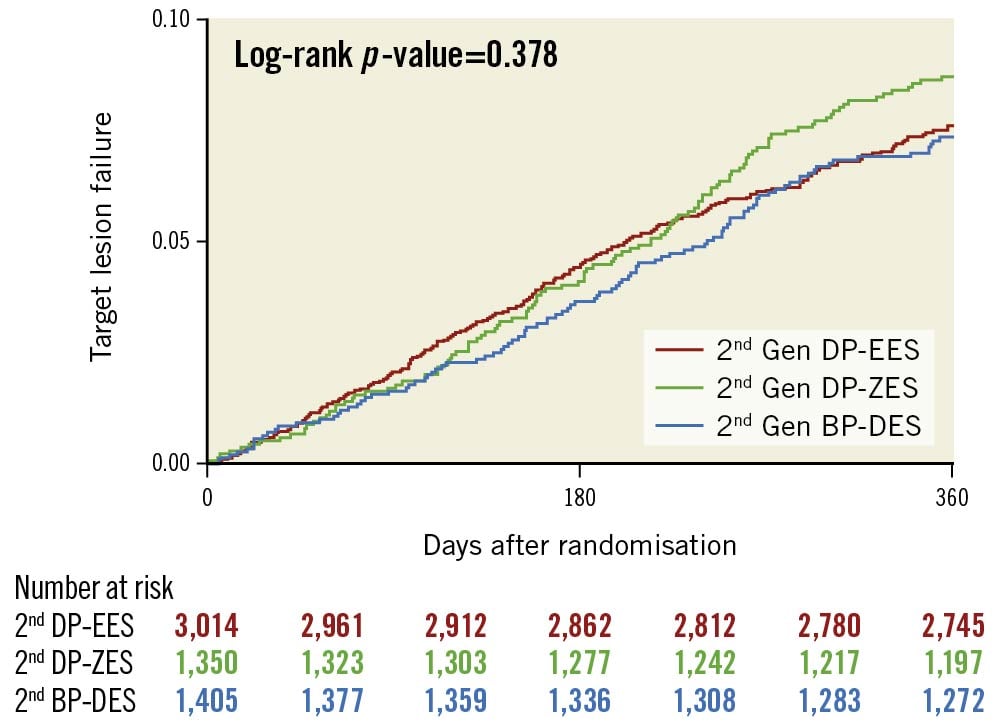
Figure 2. Rates of target lesion failure among the three DES types evaluated. Kaplan-Meier estimates for target lesion failure at 12 months after randomisation (intention-to-treat population) by drug-eluting stent type in patients who underwent percutaneous coronary intervention. BP-DES: biodegradable polymer drug-eluting stent; DP-EES: durable polymer everolimus-eluting stent; DP-ZES: durable polymer zotarolimus-eluting stent; Gen: generation
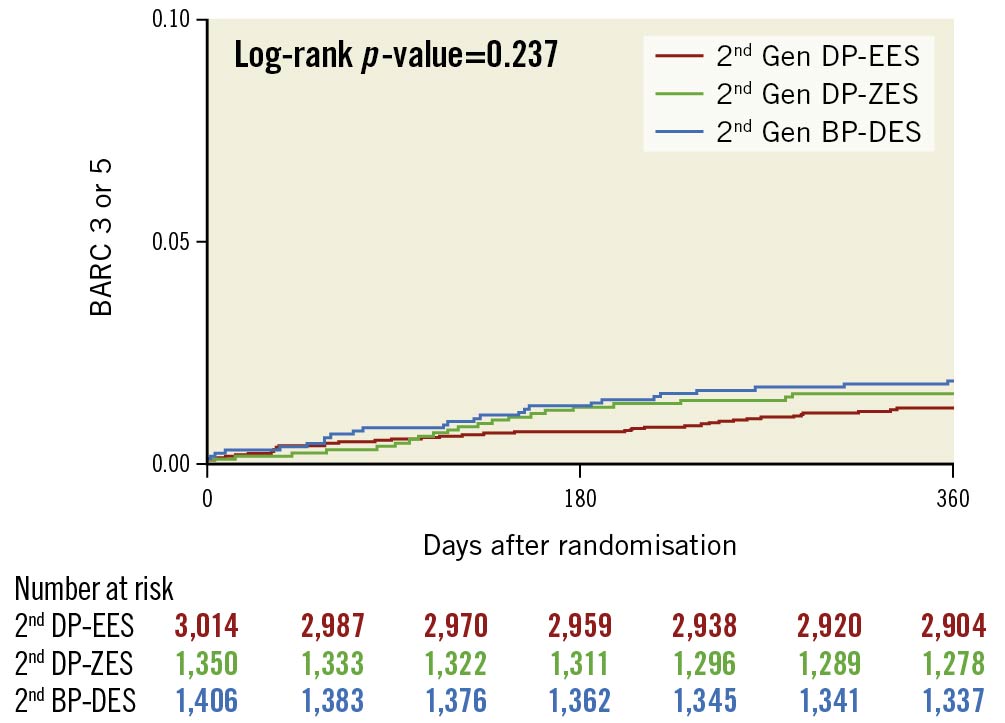
Figure 3. Rates of BARC 3 or 5 bleeding among the three DES types evaluated. Kaplan–Meier estimates for BARC 3 or 5 bleeding at 12 months after randomisation by drug-eluting stent type in patients who underwent percutaneous coronary intervention. BARC: Bleeding Academic Research Consortium; BP-DES: biodegradable polymer drug-eluting stent; DP-EES: durable polymer everolimus-eluting stent; DP-ZES: durable polymer zotarolimus-eluting stent; Gen: generation
Bleeding outcomes
Bleeding event rates in patients according to randomised treatment assignment (ticagrelor plus placebo versus ticagrelor plus aspirin) and DES type are reported in Table 2. Overall, the reduction in bleeding rates of ticagrelor monotherapy was consistent across DES types. Ticagrelor monotherapy consistently resulted in significantly lower rates of BARC type 2, 3 or 5 bleeding at one year after randomisation among patients treated with DP-EES (3.8% vs 6.7%; absolute risk difference −2.9%; HR: 0.56, 95% CI: 0.41-0.78), DP-ZES (4.6% vs 6.9%; absolute risk difference −2.3%; HR: 0.66, 95% CI: 0.42-1.04) and BP-DES (4.2% vs 7.9%; absolute risk difference −3.7%; HR: 0.52, 95% CI: 0.33-0.81), without statistical interaction (pinteraction=0.76) (Central illustration). These results were also consistent when other bleeding definitions were examined (Table 2).
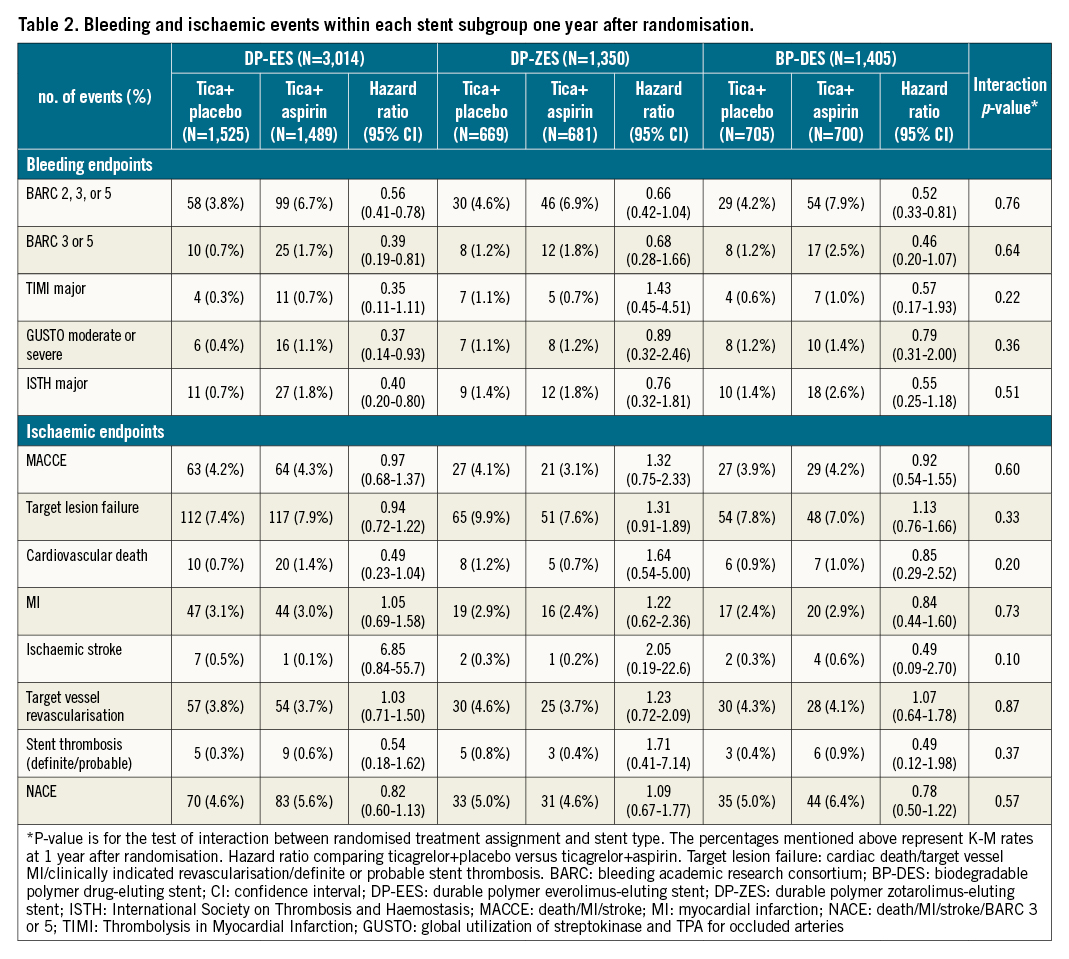
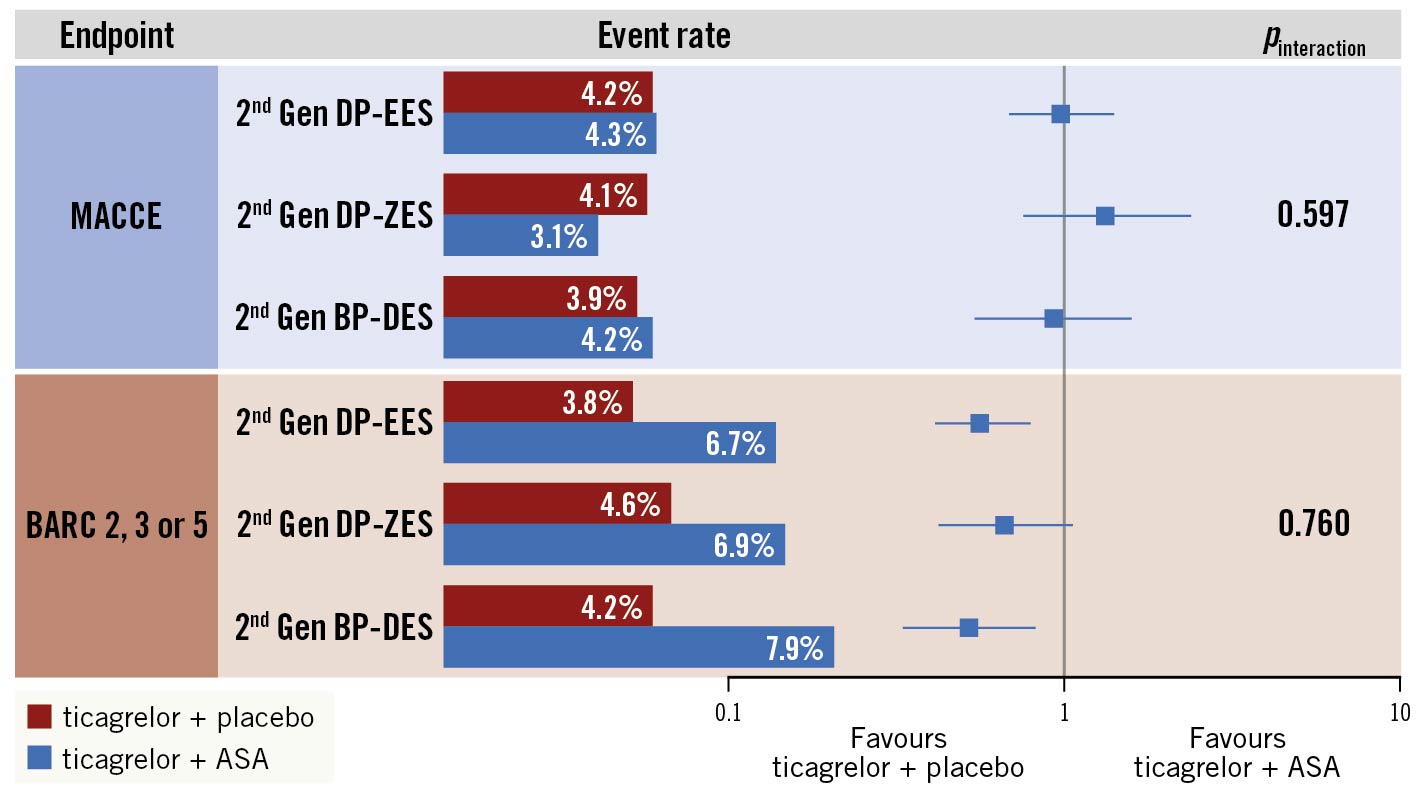
Central illustration. Bleeding and ischaemic effects of ticagrelor monotherapy versus ticagrelor plus aspirin after three months of DAPT in patients undergoing PCI with second-generation DES. Following three months of adherence to DAPT post-PCI and in the absence of major bleeding or ischaemic events, this post hoc analysis from the TWILIGHT trial assessing clinical outcomes in n=5,769 patients who underwent PCI with a second-generation DES showed that ticagrelor monotherapy, compared with ticagrelor plus aspirin, was associated with a reduction in BARC 2, 3, or 5 bleeding over one year consistently across the three studied DES types. There was no significant difference in the one-year rate of all-cause death, MI, or stroke between the two treatment arms; this was also consistent across the 3 DES types. ASA: aspirin; BARC: Bleeding Academic Research Consortium; BP-DES: biodegradable polymer drug-eluting stent; DP-EES: durable polymer everolimus-eluting stent; DP-ZES: durable polymer zotarolimus-eluting stent; Gen: generation; MACCE: major adverse cardiac and cerebrovascular events, a composite of death, myocardial infarction or stroke.
Ischaemic outcomes
Ischaemic event rates in patients according to randomised group (ticagrelor plus placebo versus ticagrelor plus aspirin) and stent type are reported in Table 2. There were no significant differences in MACCE between ticagrelor monotherapy and ticagrelor plus aspirin among patients treated with DP-EES (4.2% vs 4.3%; absolute risk difference −0.1%; HR: 0.97, 95% CI: 0.68-1.37), DP-ZES (4.1% vs 3.1%; absolute risk difference 1.0%; HR: 1.32, 95% CI: 0.75-2.33) and BP-DES (3.9% vs 4.2%; absolute risk difference −0.3%; HR: 0.92, 95% CI: 0.54-1.55), without statistical interaction (pinteraction=0.597). Additionally, there were no significant differences among groups regarding the individual ischaemic endpoints (Central illustration, Table 2). The rates of DES thrombosis were <1% and not influenced by the randomised treatment assignment to ticagrelor monotherapy or DAPT.
Discussion
The key findings of the present, post hoc analysis from the TWILIGHT trial, in which we examined the effect of aspirin withdrawal on a background of potent P2Y12-receptor inhibition with ticagrelor after three months of DAPT according to stent type, include: (i) ticagrelor monotherapy compared with ticagrelor plus aspirin resulted in significantly lower major bleeding complications, a finding that was consistent across new-generation DES types; (ii) ticagrelor monotherapy compared to ticagrelor plus aspirin was not associated with increased risk of ischaemic events irrespective of the type of new-generation DES; and (iii) there were no significant differences in MACCEs across DES types in the overall population; notably, rates of DES thrombosis were uniformly low and not influenced by the randomised treatment assignment.
Iteration in DES technologies including improved drug release kinetics, polymer biocompatibility, and endothelialisation patterns of new-generation DES significantly overcame the limitations observed with early-generation DES410. In the era of first-generation DES, an extended period of DAPT (≥1 year) using aspirin and a P2Y12-receptor inhibitor was considered necessary in order to reduce the risk of DES-related thrombotic events10. While extended DAPT has been shown to reduce the risk of DES-related and non-DES-related ischaemic events, it may also result in higher risk of haemorrhagic complications which are strongly associated with increased risk of morbidity, mortality and healthcare costs52122. New-generation DES platforms have been associated with lower risk of DES-related thrombotic events compared to first-generation DESs therefore obviating the need for mandatory prolonged DAPT1023. In a previous large meta-analysis of randomised controlled trials investigating the efficacy and safety of longer versus shorter DAPT, the risk for ST was significantly higher using short-term DAPT in patients who received a first-generation DES (OR 3.94, 95% CI: 2.20-7.05) compared with those who received a new-generation DES (OR 1.54, 95% CI: 0.96-2.47; pinteraction=0.008)10.
In the current analysis from the TWILIGHT trial, we extended prior knowledge by evaluating the safety and efficacy of a strategy of abbreviated DAPT using aspirin and ticagrelor followed by ticagrelor monotherapy among high-risk patients undergoing PCI with different types of new-generation DESs. Overall, DP-EES, DP-ZES and BP-DES were associated with very low rates of late DES thrombosis (between three and 15 months post-PCI). Among randomised patients, a strategy of ticagrelor monotherapy did not result in increased rates of MACCE nor stent thrombosis irrespective of the type of DES implanted compared to continuing DAPT. The bleeding avoidance benefits of ticagrelor monotherapy versus DAPT were not influenced by the type of DES. These findings are overall consistent with the main results of the TWILIGHT trial as well as with prior trials evaluating a strategy of P2Y12-receptor monotherapy following abbreviated DAPT using clopidogrel11. For example, in the Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt Chromium Stent (STOPDAPT 2) trial in which 3,009 patients who underwent PCI with a cobalt-chromium EES and were randomised to one month of DAPT followed by clopidogrel monotherapy versus 12 months of DAPT with aspirin and clopidogrel, the former regimen resulted in lower rates of bleeding complications and similar rates of ischaemic events compared with one-year DAPT24. In the GLOBAL LEADERS trial, a randomised, open-label superiority trial of all-comers undergoing PCI with a bioresorbable polymer biolimus A9-eluting DES (N=15,968), aspirin plus ticagrelor was tested for one month followed by 23 months of ticagrelor monotherapy and also resulted in similar rates of ischaemic events compared to 12 months of DAPT (aspirin plus clopidogrel in those with stable CAD or 12 months of aspirin plus ticagrelor in those with ACS) followed by 12 months of aspirin monotherapy25. Hence, the totality of evidence supports the efficacy and safety of a strategy of P2Y12-receptor monotherapy following an initial short period of DAPT when using a latest-generation DES after PCI in patients with high-risk clinical or anatomic characteristics26. Furthermore, P2Y12-receptor monotherapy has recently gained attention within the context of chronic maintenance therapy (i.e., beyond one year) after PCI. Indeed, the recently published HOST-EXAM randomised trial revealed a significant decrease in net adverse events (composite of all-cause death, MI, stroke, and BARC bleeding type 3 or greater) with clopidogrel versus aspirin monotherapy at 24-month follow-up among patients who were maintained on DAPT and remained event free for six to 18 months following PCI27. Whether ticagrelor monotherapy could similarly extend its benefits beyond the period tested in our trial warrants further investigation11.
Improvements in DES design continue to strive for biocompatibility; allowing enthothelialisation after the implantation-induced arterial trauma is a key process in coronary devices adherence to the arterial wall28. Strut material, thickness and metallic mesh configuration, polymer type and properties as well as drug type, dose and elution kinetics are all important. Notably, the TWILIGHT-pharmacodynamic study supported the rationale for safety of aspirin withdrawal, and the present study concurs that aspirin can be withdrawn relatively safely after an initial three-month DAPT treatment after the index PCI, irrespective of DES type29.
Limitations
Our findings should be considered in the light of the following limitations. First, as a subgroup analysis from a randomised controlled trial, the current findings can only be considered hypothesis generating and should be further tested in adequately-powered studies for individual stent types. Second, the three DES groups were not individually powered to draw definitive conclusions on the effect of ticagrelor monotherapy versus DAPT within each DES type; for the same reason, we could not perform landmark analyses assessing the time-dependent effect of ticagrelor monotherapy according to stent type. Nonetheless, the magnitude and direction of the effects were largely consistent with the overall trial findings. Third, due to absence of statistical correction for multiple comparisons, the chance findings related to multiple testing should be considered by the readers. Fourth, these results are not generalisable to all patients who undergo PCI due to the inclusion and exclusion criteria of our trial. The observed treatment effects are applicable only to patients who tolerated an initial three months of DAPT with ticagrelor plus aspirin without any major adverse events. Whether these findings across different new-generation DES types are generalisable to a regimen of clopidogrel or prasugrel monotherapy remains unknown. Finally, treatment with a specific type of DES was not randomly assigned. Therefore, these comparisons can be subject to residual confounding despite multivariable adjustment.
Conclusions
Among high-risk patients who underwent PCI, a regimen of ticagrelor monotherapy (after an initial three months of DAPT with ticagrelor plus aspirin) resulted in significantly lower clinically relevant bleeding without increasing the risk of ischaemic events compared to continuing DAPT regardless of the type of new-generation DES implanted. There were no significant differences in the rates of MACCE among types of DES between three and 15 months. Rates of stent thrombosis were low (<1%) and not influenced by the randomised assignment to ticagrelor monotherapy or DAPT.
Impact on daily practice
Owing to significant advances in DES technologies and antithrombotic therapies, initiation of ticagrelor monotherapy after three-month DAPT reduced bleeding without increasing ischaemic events as compared with 12-month DAPT across different DES types. Further studies are warranted to investigate whether shorter DAPT durations (i.e., <3 months) with ticagrelor monotherapy are a safe bleeding avoidance strategy in patients receiving different types of newer-generation DES.
Funding
Investigator-initiated grant from AstraZeneca; TWILIGHT ClinicalTrials.gov number, NCT02270242.
Conflict of interest statement
G. Dangas reports receiving consulting fees and advisory board fees from AstraZeneca, consulting fees from Biosensors, and previously holding stock in Medtronic. U. Baber reports speaker honoraria from AstraZeneca and Boston Scientific. S. Sharma has received consulting fees or honoraria from Abbott, Boston Scientific, Abiomed, and Cardiovascular Systems, Inc. S. Mehta has received research grants to the institution from AstraZeneca, Abbott, Boston Scientific, and Sanofi; and has received honoraria for consultancy from AstraZeneca, Bayer, Biosensors, and Sanofi. D. Cohen reports receiving grant support, paid to his institution, and consulting fees from AstraZeneca, Medtronic, Abbott Vascular, and Boston Scientific. D. Angiolillo has received payment as an individual for: a) Consulting fee or honorarium from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company; b) participation in review activities from CeloNova, and St. Jude Medical. He has also received institutional payments for grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli-Lilly, Gilead Sciences, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, RenalGuard Solutions, and the Scott R. MacKenzie Foundation. J. Escaned reports receiving consulting fees and lecture fees from Abbott, Philips, Boston Scientific, and Medtronic, and lecture fees from Abiomed, Terumo, and Biosensors. K. Huber reports receiving lecture fees from AstraZeneca and Bayer. V. Kunadian has received personal fees/honoraria from Bayer, Astra Zeneca, Abbott, Amgen, and Daichii Sankyo. D. Moliterno reports grants from AstraZeneca, during the conduct of the study. M. Ohman reports research grants from Abiomed and Chiesi, and consulting fees from AstraZeneca, Cara Therapeutics, Faculty Connection, Imbria, Impulse Medical, Janssen Pharmaceuticals, Milestone Pharmaceuticals, Xylocor, and Zoll Medical. G. Weisz reports receiving grant support and advisory board fees from and holding equity in Corindus, advisory board fees from and holding equity in Filterlex, serving on an advisory board for and holding options in Trisol, and receiving grant support from Abbott, CSI, and RenalGuard Solutions. M. Krucoff reports grants and/or personal fees from Abbott Vascular, Biosensors, Boston Scientific, Celonova, Medtronic, OrbusNeich, and Terumo. K. Oldroyd reports receiving grant support and lecture fees from AstraZeneca; and is employed by Biosensors. G. Sardella reports receiving consulting fees from Abbott, Shockwave, Boston Scientific, and Balmed, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbott, Alvimedica, Shockwave, Medtronic, Biosensors. P.G. Steg reports receiving research grants from Amarin, Bayer, Sanofi, and Servier; compensation for work in clinical trials from Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Idorsia, Novartis, Pfizer, Sanofi, and Servier; receiving fees for consulting or speaking from Amgen, BMS/Myokardia, Novo-Nordisk, and Regeneron; and being a Senior Associate Editor at Circulation. M. Gibson reports receiving grant support and consulting fees from Angel Medical, Bayer, CSL Behring, Janssen Pharmaceuticals, Johnson & Johnson, and Portola Pharmaceuticals; consulting fees from the Medicines Company, Eli Lilly, Gilead Sciences, Novo Nordisk, WebMD, UpToDate Cardiovascular Medicine, Amarin Pharma, Amgen, Boehringer Ingelheim, Chiesi, Merck, PharmaMar, Sanofi, Somahlution, Verreseon Corporation, Boston Scientific, Impact Bio, MedImmume, Medtelligence, MicroPort, PERT Consortium, and GE Healthcare, holding equity in Nference; serving as chief executive officer of Baim Institute; and receiving grant support, paid to Baim Institute, from Bristol-Myers Squibb. R. Mehran reports institutional research grants from Abbott, Abiomed, Applied Therapeutics, Arena, AstraZeneca, Bayer, Biosensors, Boston Scientific, Bristol-Myers Squibb, CardiaWave, CellAegis, CERC, Chiesi, Concept Medical, CSL Behring, DSI, Insel Gruppe AG, Medtronic, Novartis Pharmaceuticals, OrbusNeich, Philips, Transverse Medical, and Zoll; personal fees from ACC, Boston Scientific, California Institute for Regenerative Medicine (CIRM), Cine-Med Research, Janssen, WebMD, SCAI; consulting fees paid to the institution from Abbott, Abiomed, AM-Pharma, Alleviant Medical, Bayer, Beth Israel Deaconess, CardiaWave, CeloNova, Chiesi, Concept Medical, DSI, Duke University, Idorsia Pharmaceuticals, Medtronic, Novartis, and Philips; equity <1% in Applied Therapeutics, Elixir Medical, STEL, and CONTROLRAD (spouse); being on the scientific advisory board for AMA, and Biosensors (spouse). The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

