
Abstract
Evidence from studies published more than 10 years ago suggested that patients receiving first-generation drug-eluting stents (DES) needed dual antiplatelet therapy (DAPT) for at least 12 months. Current evidence from randomised controlled trials (RCT) reported within the past five years suggests that patients with stable ischaemic heart disease who receive newer-generation DES need DAPT for a minimum of three to six months. Patients who undergo stenting for an acute coronary syndrome benefit from DAPT for at least 12 months, but a Bayesian network meta-analysis confirms that extending DAPT beyond 12 months confers a trade-off between reduced ischaemic events and increased bleeding. However, the network meta-analysis finds no credible increase in all-cause mortality if DAPT is lengthened from three to six months to 12 months (posterior median odds ratio [OR] 0.98; 95% Bayesian credible interval [BCI]: 0.73-1.43), from 12 months to 18-48 months (OR 0.87; 95% BCI: 0.64-1.17), or from three to six months to 18-48 months (OR 0.86; 95% BCI: 0.63-1.21). Future investigation should focus on identifying scoring systems that have excellent discrimination and calibration. Although predictive models should be incorporated into systems of care, most decisions about DAPT duration will be based on clinical judgement and patient preference.
Abbreviations
ACS: acute coronary syndrome
ARCTIC-Interruption: Assessment by a double Randomisation of a Conventional antiplatelet strategy versus a monitoring-guided strategy for drug-eluting stent implantation and, of Treatment Interruption versus Continuation 1 year after stenting
BES: biolimus-eluting stent
BMS: bare metal stent(s)
CAD: coronary artery disease
CAPRIE: a randomised, blinded, trial of Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events
CHARISMA: Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance
CLASSICS: Clopidogrel Aspirin Stent International Cooperative Study
CREDO: Clopidogrel for the Reduction of Events During Observation
CURE: Clopidogrel in Unstable angina to prevent Recurrent Events
DAPT: dual antiplatelet therapy
DES: drug-eluting stent(s)
DES-LATE Optimal Duration of Clopidogrel Therapy with DES to Reduce Late Coronary Arterial Thrombotic Events
EXCELLENT: Efficacy of Xience/Promus Versus Cypher in rEducing Late Loss After stENTing
FANTASTIC: Full Anticoagulation Versus Aspirin and Ticlopidine
I-LOVE-IT 2 Evaluate Safety and Effectiveness of the Tivoli® DES and the Firebird DES for Treatment of Coronary Revascularization
ISAR: Intracoronary Stenting and Antithrombotic Regimen study
ISAR-SAFE Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting
ITALIC: Is There A LIfe for DES after discontinuation of Clopidogrel
IVUS-XPL Impact of Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions
LEADERS-FREE Prospective Randomised Comparison of the BioFreedom Biolimus A9 Drug-Coated Stent Versus the Gazelle Bare-Metal Stent in Patients at High Bleeding Risk
MACCE: major adverse cardiac and cerebrovascular events
MATTIS: Multicentre Aspirin and Ticlopidine Trial after Intracoronary Stenting
MI: myocardial infarction
NIPPON: Nobori Dual Antiplatelet Therapy as Appropriate Duration
OPTIDUAL: OPTImal DUAL antiplatelet therapy after drug-eluting stent implantation
OPTIMIZE: Optimised Duration of Clopidogrel Therapy Following Treatment with the Endeavor Zotarolimus-Eluting Stent in Real-World Clinical Practice
PCI: percutaneous coronary intervention
PRODIGY: PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY
RCT: randomised controlled trial(s)
RESET: REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation
SECURITY: Second-generation Drug-eluting Stent Implantation Followed by 6- versus 12-month dual antiplatelet therapy
ST: stent thrombosis
STARS: Stent Anticoagulation Restenosis Study
ZEUS: Zotarolimus-eluting Endeavor sprint stent in Uncertain DES candidates
Introduction
In its 40th anniversary, percutaneous coronary intervention (PCI) has achieved excellent early and late outcomes thanks to advances in technologies, operator expertise, and antithrombotic therapy. Although the advent of drug-eluting stents (DES) has been crucial for the overall success of PCI, stent thrombosis (ST) and myocardial infarction may occur unless patients adhere to a strict regimen of dual antiplatelet therapy (DAPT), which consists of concurrent use of aspirin and a P2Y12 platelet receptor blocker. The use of DAPT, however, confers an increased risk of major bleeding that in some instances is fatal.
The purpose of the current report is to review the early developments that have led to replacement of anticoagulation therapy with DAPT after stent implantation, current recognition of the prognostic significance of major bleeding, and ultimate awareness that the duration of DAPT after DES implantation must be prescribed on an individual basis.
The past
On 16 September 1977, Andreas Grüntzig performed the first coronary balloon angioplasty in a 37-year-old man with a proximal stenosis in the left anterior descending artery. The result was successful and durable1,2. However, in the series of 624 patients undergoing coronary angioplasty between 1977 and 1981 in Zurich and Atlanta, emergency operations due to sudden closure or spasm of the artery occurred in 5% and Q-wave myocardial infarction (MI) in 3%, but no in-hospital deaths occurred1. At that time, the optimal pharmacotherapy to prevent failure and complications remained uncertain3. Early investigators recommended the use of warfarin as long-term adjunctive therapy after femoropopliteal transluminal angioplasty and its use was also adopted for the treatment of acute MI, whereas other studies demonstrated benefit from the administration of aspirin after MI4. In a randomised comparison of aspirin and coumadin in 248 PCI patients, aspirin did not reduce recurrent stenoses as compared with coumadin at nine months of follow-up (27% vs. 36%; p=not significant)4.
In subsequent studies, evidence supported benefits of aspirin therapy after MI or PCI5, but at the same time balloon angioplasty seemed to be limited by a high incidence of abrupt vessel closure after dilatation and requirement for reintervention for restenosis. The implantation of an expandable metal stent to maintain vessel patency after balloon dilatation emerged as the solution to these problems6,7. Nevertheless, the inherent thrombogenicity of metal stents that were in contact with circulating blood resulted in thrombotic stent occlusion despite aggressive anticoagulant therapy. In a pivotal trial, angiographic follow-up after placement of a self-expanding coronary artery stent showed that early occlusion occurred in approximately 20% of cases8. Additionally, haemorrhagic and peripheral vascular complications due to the intensive anticoagulation adopted for the first few weeks after the procedure seriously limited the benefits of PCI.
Two studies in 1995 were the first to suggest that the combination of aspirin and ticlopidine was a safe replacement for anticoagulant therapy after coronary stent implantation9,10. In 1996, ISAR suggested advantages of DAPT over anticoagulation by showing that combined antiplatelet therapy (aspirin plus ticlopidine) after the placement of coronary stents reduced the incidence of both cardiac events and haemorrhagic and vascular complications compared with conventional anticoagulation-based therapy (intravenous heparin, phenprocoumon, and aspirin)11. Later, STARS demonstrated that DAPT was superior to anticoagulant therapy after implantation of bare metal stents (BMS) and reduced ST by 85% as compared with aspirin alone12. In the FANTASTIC study, which studied both elective and unplanned coronary stenting, DAPT with aspirin and ticlopidine significantly reduced rates of bleeding and subacute stent occlusion compared with conventional anticoagulation13. In the MATTIS study, high-risk patients receiving aspirin and ticlopidine after coronary stenting had significantly reduced bleeding and vascular complications and there was a marked trend towards decreased cardiac events compared with aspirin and anticoagulation14. At the same time, clopidogrel appeared, which was a new thienopyridine derivative that had fewer side effects than ticlopidine. The CAPRIE trial suggested that clopidogrel could be used in place of aspirin to prevent ischaemic stroke, MI or vascular death in patients at risk of ischaemic events15. Then the CLASSICS study supported replacement of ticlopidine by clopidogrel after coronary stenting due its safer profile16. The CURE study confirmed the efficacy and safety of clopidogrel added to aspirin in patients with ACS and in those undergoing PCI17,18.
Evidence from early studies thus suggested that a strategy based on aspirin and a thienopyridine was substantially more effective and better tolerated than anticoagulation, thus facilitating a widespread adoption of stenting in clinical practice. Indeed, the last two decades have established the pivotal role of DAPT in preventing both stent- and non-stent-related ischaemic events after PCI compared with single antiplatelet therapy or anticoagulation. Recently, new hypotheses have been studied or are still under evaluation (i.e., very short DAPT regimens and aspirin interruption during follow-up)5. However, the optimal duration of DAPT after stent implantation has been a matter of contention for years. Indeed, in parallel with the evolution of the DAPT regimens, stent technology has evolved from BMS to first-generation DES, a change that has implications for DAPT regimens. When clopidogrel was approved in 1997 by the U.S. Food and Drug Administration (FDA), it was recommended for two weeks after BMS implantation19 and then later for four weeks16. When sirolimus-eluting stents were approved in 2003, the labelling recommended three months of clopidogrel because that is how the agent had been used in clinical trials20. When paclitaxel-eluting stents were approved in 2004, the labelling recommended six months of clopidogrel, again based on how it had been used in trials21. Later, this DAPT duration was seriously questioned due to increasing safety concerns that were initially related to late and very late ST in first-generation DES, but also to an increase in death and MI. Indeed, 2006 was a critical year for evidence on first-generation DES, but an expert FDA panel concluded that DES appeared to increase the risk of late stent thrombosis, but not the risk of death or MI22. At that time, the concerns about DES led to the empirical recommendation of 12 months of DAPT. The panel also agreed on the urgent need for studies on ST and the duration of DAPT22.
The recommendation of 12 months of DAPT was maintained in the following few years with the sole exception of patients in whom the risk of bleeding outweighed the anticipated benefit. Based on previous findings from the PCI-CURE (stenting comprised 80% of the PCI cases, but all stents were BMS and the mean duration of DAPT was nine months) and CREDO studies (all BMS; only 63% of patients assigned to clopidogrel finished one year of therapy)18,23, and observational studies reporting a persistent risk of ST beyond six months after stenting, particularly in the context of DAPT cessation24-26, the 2011 American guideline recommended a minimum DAPT duration of at least 12 months after DES implantation27. The European guidelines in 2010 recommended one month of DAPT after BMS in stable patients, but six to 12 months after DES, and 12 months in the case of ACS28. An additional relevant milestone of DAPT history was the introduction of the new P2Y12 inhibitors, prasugrel in 200729 and ticagrelor in 200930, which further improved outcomes of ACS patients undergoing PCI and receiving DAPT.
Figure 1 shows the main steps in the advent and evolution of DAPT, and Figure 2 shows the mechanism of action of DAPT.
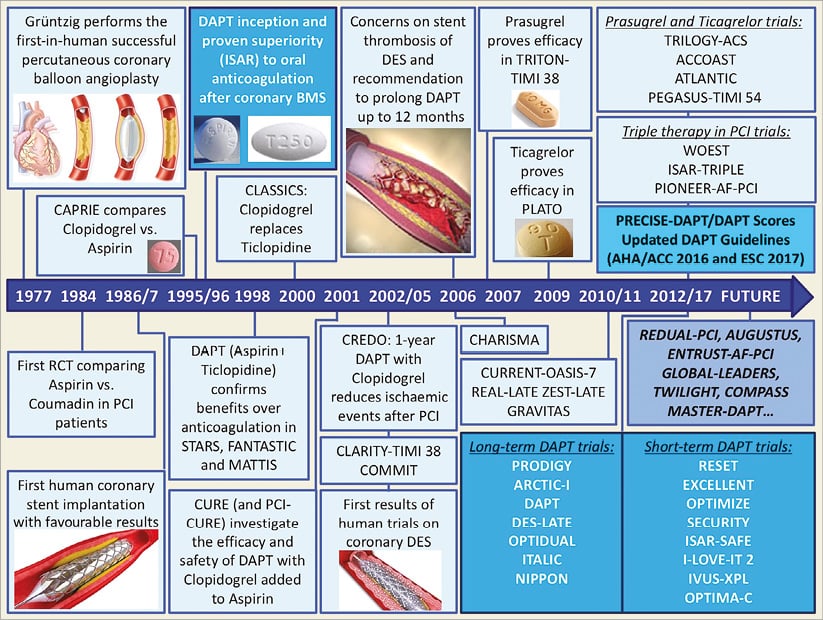
Figure 1. History of DAPT in PCI.
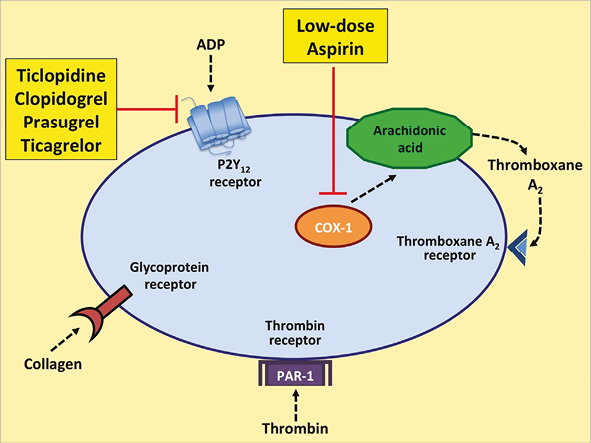
Figure 2. Sites of action of DAPT. DAPT includes aspirin, a cyclooxygenase-1 (COX-1) inhibitor, and a P2Y12 receptor inhibitor (ticlopidine, clopidogrel, prasugrel, or ticagrelor). ADP: adenosine diphosphate; PAR-1: protease-activated receptor-1
The present
GUIDELINES, TRIALS AND META-ANALYSES OF DAPT DURATION
The guidelines from the European Society of Cardiology recommend at least one month of DAPT for stable ischaemic heart disease (SIHD) treated with BMS and at least six months if treated with DES, while in ACS patients a 12-month DAPT was recommended, suggesting that shorter courses in patients with SIHD or longer courses in patients with a history of ACS may be considered31,32. A 2016 focused update on DAPT from the American College of Cardiology/American Heart Association33 recommended a minimal mandatory duration of DAPT of six months after implantation of newer-generation DES in patients with SIHD and replaced the 2011 guideline recommendation of at least 12 months27. The abbreviated course of therapy for patients with SIHD seemed reasonable, because the risk of ST with newer-generation DES was lower than it was with first-generation DES34.
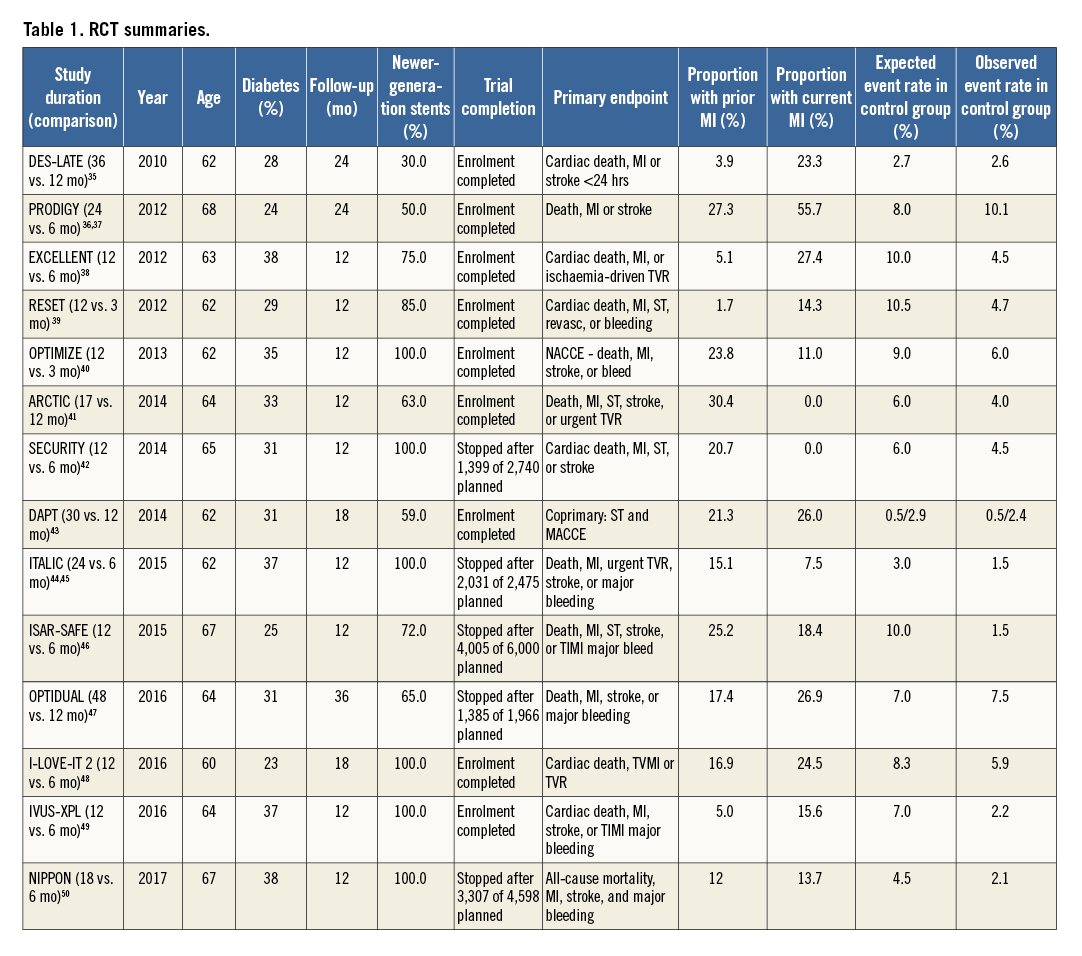
Since publication of the DAPT update, new evidence regarding DAPT duration has emerged. Data from 14 RCT (Figure 3) of patients undergoing implantation of DES, with more than two thirds of subjects receiving newer-generation stents (Table 1), and randomised to either prolonged or short-course DAPT, have been published35-50. The largest RCT of DAPT duration, the DAPT trial43, randomly assigned 9,961 patients to prolonged DAPT of 2.5 years or to short-course DAPT of 12 months after DES implantation. Prolonged DAPT was associated with a reduced rate of ST (0.4% vs. 1.4%; HR 0.29, 95% CI: 0.17-0.48, p<0.001), MACCE (4.3% vs. 5.9%; HR 0.71, 95% CI: 0.59-0.85, p<0.001) and reduced MI (2.1% vs. 4.1%, HR 0.47, p<0.001) but was associated with a borderline increased risk of death from any cause (2.0% vs. 1.5%, HR 1.36, 95% CI: 1.00-1.85, p=0.05) and increased moderate or severe bleeding (2.5% vs. 1.6%, p=0.001).
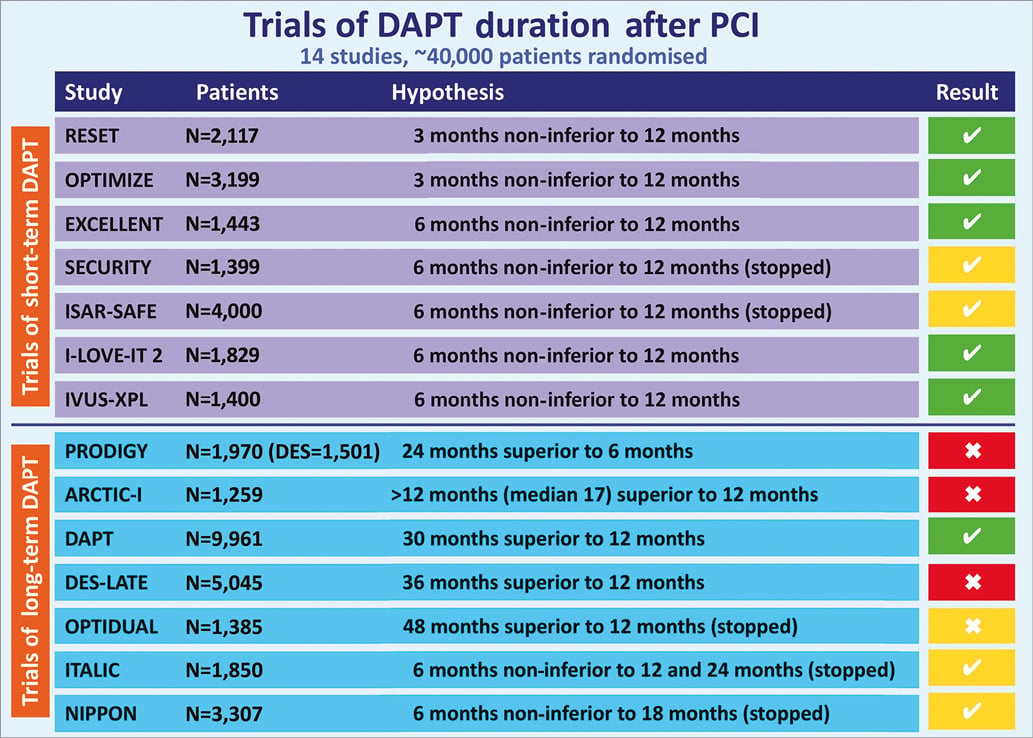
Figure 3. Trials of DAPT after PCI. Trial result is reported according to whether the hypothesis was demonstrated (![]() , green colour) or not (
, green colour) or not (![]() , red colour). Five trials are reported with yellow colour due to premature interruption of planned enrolment.
, red colour). Five trials are reported with yellow colour due to premature interruption of planned enrolment.
When aggregate data from the 14 RCT including the DAPT trial are pooled (Appendix), short compared with prolonged DAPT is associated with no significant difference in mortality (odds ratio [OR] 0.85; 95% CI: 0.72-1.01) and reduced major bleeding (OR 0.68, 95% CI: 0.55-0.82). On the other hand, as shown in Figure 4, shorter courses of DAPT are associated with more cases of MI (OR 1.37, 95% CI: 1.12-1.67) and ST (OR 1.69, 95% CI: 1.13-2.54).
The absence of a mortality benefit from prolonged DAPT may seem counterintuitive given the reductions in MI and ST, but these findings may reflect a temporal attenuation in mortality risk attributable to ST. While acute and subacute ST are associated with mortality rates approaching 50%, late and very late ST are associated with mortality rates of about 10%51. As a result, it is possible that extension of DAPT beyond 12 months may simultaneously reduce both MI and ST without influencing mortality. On the other hand, major bleeding may be more dangerous than non-fatal MI52-57. Taken together, the reductions in mortality from lowering thrombosis with prolonging DAPT may be counterbalanced by an increase in mortality from bleeding complications58.
Although a large number of meta-analyses of the DAPT RCT have been published37,59-63, they have produced mixed results. Apparent discrepancies may have arisen because traditional meta-analyses comparing outcomes use a binary short-versus-long definition of DAPT duration. This poses a problem, even for the traditional meta-analysis presented here (Figure 4), because 12 months of DAPT was defined as “short” in four trials35,41,43,47 and as “long” in seven trials38-40,42,46,48,49. Comparing outcomes at 12 months with outcomes at 12 months in a meta-analysis may unintentionally introduce noise in the statistical models. An alternative approach is to avoid 12-month versus 12-month comparisons, as was done by Navarese and colleagues in a stratified meta-analysis62, but a Bayesian network meta-analysis may take advantage of the complete evidence base and provide an optimal approach to compare outcomes after short (three to six months), intermediate (12 months), and prolonged (18-48 months) durations of DAPT.
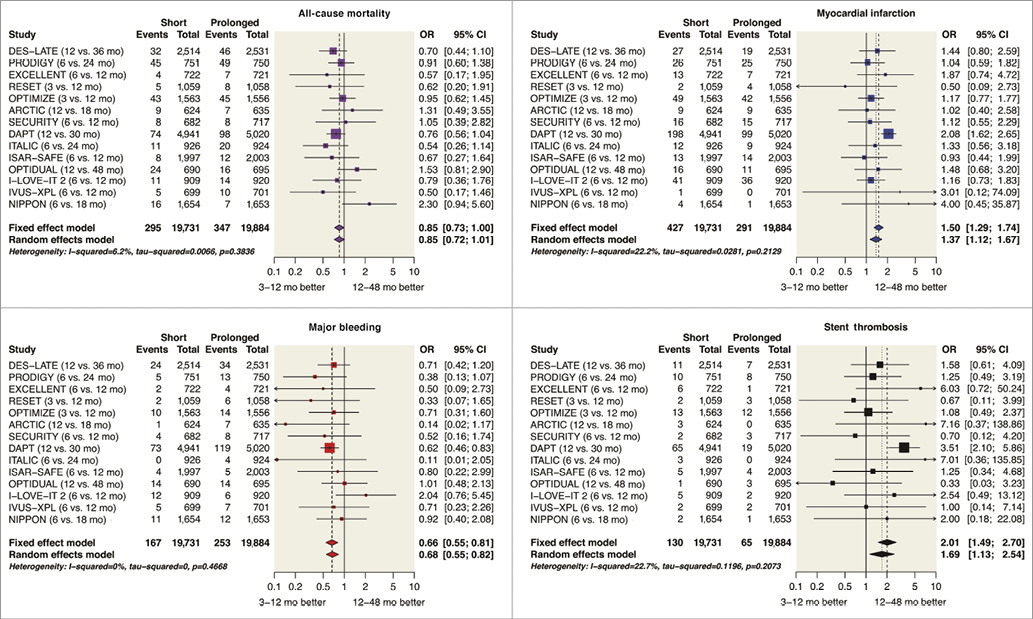
Figure 4. Forest plot of event rates after prolonged or short course of DAPT after drug-eluting stent implantation. Original figures created with the open-source statistical program [R] 3.0.385 and library package “meta” 3.8-086. Note: OPTIMA-C trial (6 vs. 12-month DAPT) was completed in 2015, presented orally in 2015 but not yet published, recently registered on clinicaltrials.gov (NCT03056118), and therefore not included here. Adapted with permission from the American Heart Association87. CI: confidence interval; OR: odds ratio
The use of network meta-analysis clarifies the differences in outcomes after short durations of three to six months, the standard comparator of 12 months, and prolonged durations of 18-48 months of DAPT (Figure 5) and reveals no credible reductions in mortality when DAPT was used for three to six months as compared with 12 months (posterior OR 0.98; 95% Bayesian credible interval [BCI]: 0.73-1.43), when DAPT was used for 12 months as compared with 18-48 months (OR 0.87; 95% BCI: 0.64-1.17), or when DAPT was used for three to six months as compared with 18-48 months (OR 0.86, 95% BCI: 0.63-1.21). Moreover, no difference in any major outcome was seen between three to six months and 12 months of DAPT, but bleeding was lower when DAPT was used for three to six months as compared with 18-48 months (OR 0.53, 95% BCI: 0.33-0.81), a finding that is counterbalanced by increased MI (OR 1.72, 95% BCI: 1.18-2.42) and ST (OR 2.56, 95% BCI: 1.23-5.03).
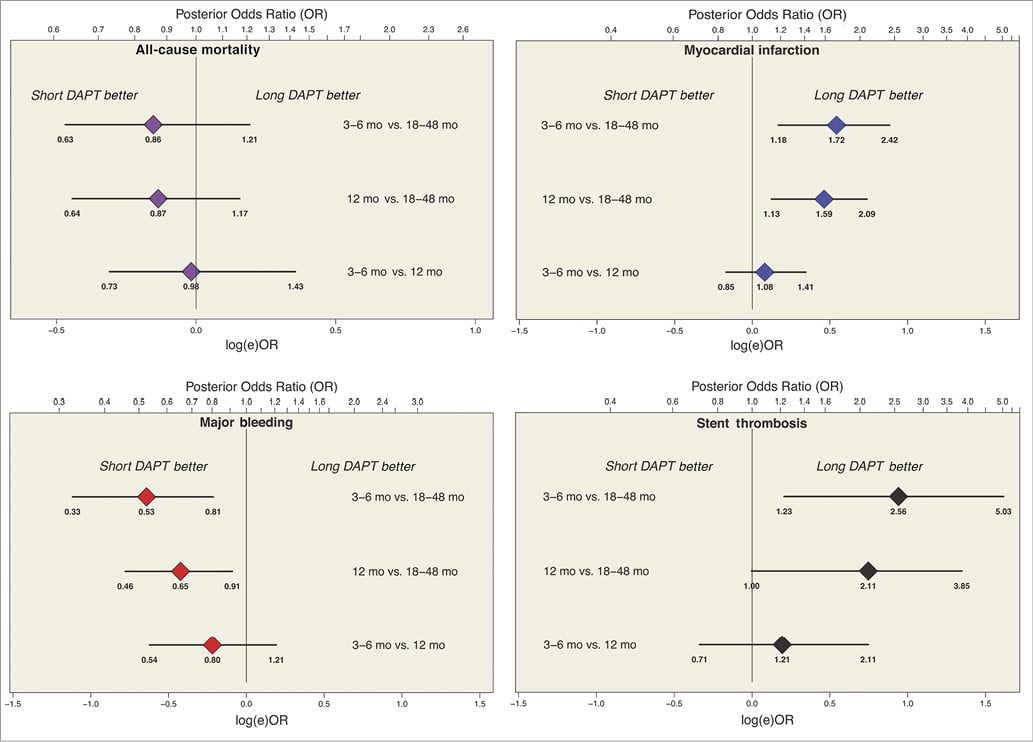
Figure 5. Caterpillar plot of event rates and duration of DAPT. In a network meta-analysis, the number of events after each duration of DAPT was modelled using a binomial distribution, and the logit of each rate had a non-informative prior distribution to ensure that the posterior inference would be dominated by the likelihood of the data. Data presented as posterior mean odds ratio and 95% Bayesian credible intervals. Original figures were created with OpenBUGS (Bayesian inference using Gibbs sampling) and Markov chain Monte Carlo modelling, starting with non-informative priors centred at 0.000 with precision of 0.0001 and using 10,000 draws of the Gibbs chain, to ensure that the posterior distribution would be dominated by the likelihood, using described methods (Figure 7)85,87-89. Adapted with permission from the American Heart Association87
ISCHAEMIC AND BLEEDING RISKS OF DAPT AND DECISION MAKING ON DAPT DURATION
DAPT with aspirin and a P2Y12 inhibitor reduces ischaemic recurrences but increases bleeding risk, which is related to the treatment duration. It is now clear that both ischaemic and bleeding risks can negatively impact on prognosis52-58. Therefore, the decision as to whether DAPT should be continued beyond one year after PCI requires preliminary clarification of the relative weight of ischaemic and bleeding events on mortality. Choosing between these two negative outcomes with similar frequencies and prognostic implications remains a great challenge. Tailored treatment algorithms maximising benefits over risks represent the only sensible way forward.
Some subgroups of patients undergoing DES implantation may benefit from extending DAPT, such as patients with prior MI64,65, ACS at presentation66,67, complex PCI68 or peripheral arterial disease69,70. On the other hand, other patient characteristics may not benefit from extending DAPT, such as diabetes71, chronic kidney disease72, or advanced age73. In patients with high bleeding risk, a course of DAPT as short as one month has been found to be feasible74,75.
Against this background, recently proposed tools derived from randomised studies, namely the DAPT and PRECISE-DAPT scores76,77, may help to guide the decision making. The DAPT score was proposed for patients who tolerated 12 months of DAPT to select those eligible for treatment prolongation78. It was derived from 11,648 patients randomised in the entire DAPT database and is based on ischaemic and bleeding risk factors to help identify patients with greater expected benefit versus greater expected harm from prolonging DAPT over one year after stenting. It assigns 1 point each for MI at presentation, prior MI or PCI, diabetes, stent diameter less than 3 mm, smoking, and paclitaxel-eluting stent; 2 points each for history of congestive heart failure/low ejection fraction and vein graft intervention; −1 point for age 65 to 74 years; and −2 points for age ≥75 years. In patients with clinical predictive scores of 2 or higher, continued thienopyridine therapy was associated with an absolute risk reduction in MI or ST that was 8.2 times greater than the absolute risk increase in moderate or severe bleeding. On the other hand, among patients with scores lower than 2, DAPT prolongation was associated with an absolute increase in bleeding that was 2.4 times the absolute reduction in MI or ST79. Of note, the DAPT score is only applicable to patients who have completed one year of DAPT after coronary stent treatment without a major ischaemic or bleeding event and cannot be applied earlier, at the time of PCI, to select less than 12 months of treatment in patients at high bleeding risk.
More recently, a novel risk score (PRECISE-DAPT) has been proposed for the prediction of out-of-hospital bleeding in patients treated with DAPT using age, creatinine clearance, white blood cell count, haemoglobin, and history of bleeding77. High bleeding risk patients (score ≥25) can be easily detected and might benefit from a shortened (i.e., <12 months) DAPT duration. Conversely, patients not at high bleeding risk (score <25) might receive a standard (i.e., 12 months) or prolonged (i.e., >12 months) treatment without being exposed to significant bleeding liability. The PRECISE-DAPT score is a simple bedside risk assessment tool, which can be easily implemented in everyday clinical practice and might be useful at the time of treatment initiation. A suggested algorithm for decision making based on these two scores is shown in Figure 6.
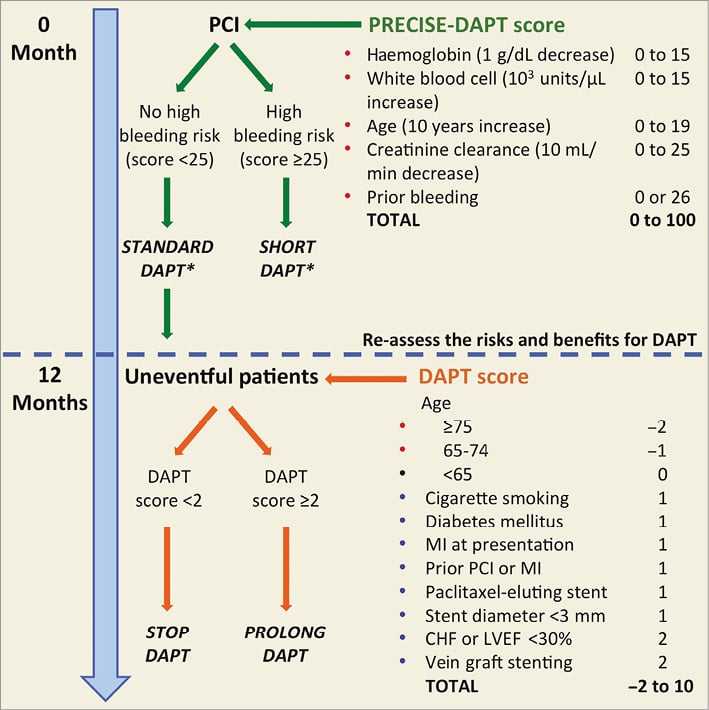
Figure 6. Decision making on DAPT duration based on PRECISE-DAPT and DAPT scores. Variables included in the scores are associated with increased bleeding risk (red dot), increased ischaemic risk (blue dot) or neutral effect (black dot). *In the validation study, short-term DAPT consisted of three to six months of therapy and standard DAPT consisted of at least 12 months of DAPT.
Authors’ perspectives
We believe that, in low-risk patients who have undergone newer-generation DES implantation, a minimum DAPT duration of three to six months is sufficient to prevent stent-related thrombotic events. On the other hand, patients at high risk of thrombotic events79 and low risk of bleeding77 may derive a benefit from extension of DAPT beyond six to 12 months. Several scoring systems have appeared, but additional prospective investigation will be required to define their utility in everyday practice80. Future studies will need to identify optimal DAPT duration in patients who receive bioresorbable scaffolds81.
In accordance with a personalised approach, patients at high bleeding risk on DAPT need special attention. The multicentre randomised open-label MASTER-DAPT trial (NCT03023020) is currently enrolling 4,300 high bleeding risk patients in >100 international centres to compare one-month DAPT with a more prolonged regimen consisting of at least three or six months of DAPT depending on whether the patient has or has not a concomitant indication to oral anticoagulation.
The future role of aspirin is also a matter of ongoing investigation5. Historical evidence comparing aspirin with placebo showed a great reduction in thrombotic risk and supports current recommendations. However, P2Y12 inhibitors have mostly been studied as adjuncts to aspirin; the comparison of single antiplatelet therapy with new P2Y12 inhibitors alone versus DAPT after ACS or PCI for secondary prevention of atherothrombotic events is a new field of research. While up-to-date research has focused on DAPT and its duration, an alternative and original approach is the “less is more” paradigm exploring the role of monotherapy with new P2Y12 inhibitors for efficacy and also for the reduction in risk of bleeding. The GLOBAL LEADERS (NCT01813435) trial is designed to assess the role of ticagrelor as a single antiplatelet agent after a short course of DAPT for the long-term prevention of adverse cardiac events, across a wide spectrum of patients, following BES implantation82.
The subject of several ongoing trials is the comparison of treatment regimens combining an oral anticoagulant (warfarin or novel oral anticoagulants) with single or dual antiplatelet therapy for patients with atrial fibrillation and ACS or coronary stents5,83,84. The role of long-term secondary prevention with novel oral anticoagulant (NOAC)-based regimens (i.e., NOAC alone or in combination with aspirin) will be re-assessed and will probably impact on our future practice. The routine use of platelet function testing or genotyping to guide clinical decisions is not currently recommended, but future evidence may eventually provide new insights on this topic.
Finally, only selected DES have received CE mark approval for one-month DAPT for patients in need; however, this was based on limited data. Whether DAPT should be stent-specific or whether the newer-generation DES have different DAPT requirements remains a matter of ongoing investigation.
Conclusions
No single DAPT recommendation applies to every patient. In low-risk patients who receive a newer-generation DES, a minimum DAPT duration of three to six months may be sufficient to prevent early and largely stent-related thrombotic events. Patients who undergo stenting for acute coronary syndrome may benefit from DAPT for at least 12 months. Extension of DAPT beyond 12 months entails a trade-off between increased bleeding and reduced ischaemic events. Because RCT can only elucidate broad principles and scoring systems only consider a small number of risk factors for bleeding or ischaemic risk, the fine details of DAPT duration must be defined by clinicians for each patient on an individual basis.
Appendix. Methods
Aggregate data from 14 randomised controlled trials (RCT) of patients undergoing implantation of predominantly newer-generation drug-eluting stents (DES) and randomised to either shorter or longer courses of dual antiplatelet therapy (DAPT)35-50 comprise the evidence base for the analysis of DAPT duration after DES implantation. As described previously63,89, data from each trial had been abstracted in duplicate by two reviewers (J.A. Bittl and U. Baber). The present review uses the previously abstracted data to create the original forest plots and caterpillar plots using procedures and data shown here (Table 2 and Figure 7).
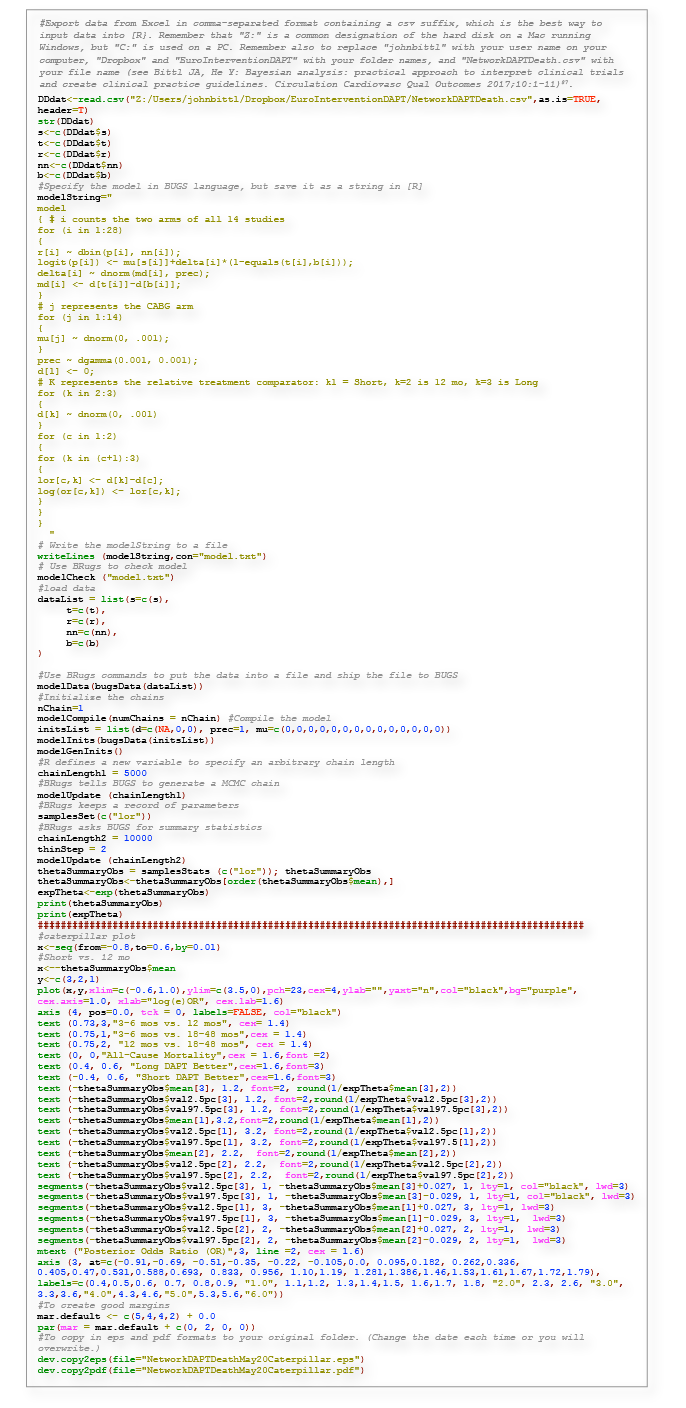
Figure 7. R Code for Figure 5: network meta-analysis for DAPT mortality and caterpillar plot.
BAYESIAN NETWORK META-ANALYSIS
Because each RCT performed two-way DAPT comparisons of DAPT durations that varied widely, an indirect three-way comparison of outcomes after short, medium or long durations of DAPT was carried out using Bayesian network meta-analysis. As described in detail, we modelled the number of deaths after short courses of DAPT in the seven studies that had both three- to six-month and 12-month arms38-40,42,46,48,49 by using a binomial distribution. We assumed that the difference of log odds between a short (S) duration of DAPT and a 12-month duration (M) of DAPT from each study δi,SM followed a normal random effects distribution with mean dSM and variance τ2SM, where dSM characterised the comparative effectiveness between a short duration of DAPT and 12 months of therapy. Similarly, we modelled the number of deaths after prolonged DAPT in the four studies that had treatment arms comparing 12 months (M) of DAPT with long (L) durations of DAPT of 18-48 months35,41,43,47,49 as a binomial distribution. We assumed that the difference of log odds from each study δi,ML followed a normal random effects distribution with mean dML and variance τ2ML, where dML characterised the comparative effectiveness between prolonged DAPT and 12 months of therapy.
The difference between dSM and dML can be denoted by dSL=dSM–dML to describe the comparative effectiveness between short and long durations of DAPT under the model. Finally, we completed the model specification by imposing the following prior distributions to the parameters:
dSM ~ N[0,103]
dML ~ N[0,103],
τ2SM ~ IG[10–3,10–3],
τ2ML ~ IG[10–3,10–3],
where d is the mean difference in the log odds of an outcome after S, M or L DAPT and τ2 is the associated variance modelled using a normal (N) or inverse gamma (IG) distribution, based on the complete model described in other reports87.
Conflict of interest statement
G. Gargiulo is supported by a research grant from Cardiopath. The other authors have no conflicts of interest to declare.

