Abstract
Aims: Our aim was to report on a survey initiated by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) concerning opinion on the evidence relating to dual antiplatelet therapy (DAPT) duration after coronary stenting.
Methods and results: Results from three randomised clinical trials were scheduled to be presented at the American Heart Association Scientific Sessions 2014 (AHA 2014). A web-based survey was distributed to all individuals registered in the EuroIntervention mailing list (n=15,200) both before and after AHA 2014. A total of 1,134 physicians responded to the first (i.e., before AHA 2014) and 542 to the second (i.e., after AHA 2014) survey. The majority of respondents interpreted trial results consistent with a substantial equipoise regarding the benefits and risks of an extended versus a standard DAPT strategy. Two respondents out of ten believed extended DAPT should be implemented in selected patients. After AHA 2014, 46.1% of participants expressed uncertainty about the available evidence on DAPT duration, and 40.0% the need for clinical guidance.
Conclusions: This EAPCI survey highlights considerable uncertainty within the medical community with regard to the optimal duration of DAPT after coronary stenting in the light of recent reported trial results. Updated recommendations for practising physicians to guide treatment decisions in routine clinical practice should be provided by international societies.
Introduction
The importance of dual antiplatelet therapy (DAPT) in patients with acute coronary syndromes and after coronary stent implantation has been substantiated in numerous trials1,2 and has also been endorsed by international guidelines3,4. However, the optimal duration of DAPT after coronary stenting, which maximises the benefits in terms of ischaemic protection and minimises the risks in terms of bleeding, remains unclear.
Between 2010 and 2014 results have been reported from a number of randomised clinical trials comparing different DAPT duration regimens after coronary stent implantation5. Data from these studies failed to show clear evidence of benefit in terms of ischaemic events, in prolonging DAPT beyond one year. Moreover, a DAPT regimen shorter than 12 months was shown to be safer than the currently recommended 12-month DAPT duration6. During the American Heart Association Scientific Sessions 2014 (AHA 2014), results from three additional clinical trials investigating the optimal DAPT duration after stenting in an aggregate of approximately 20,000 randomised patients – DAPT, ISAR-SAFE and ITALIC7-9 – were reported for the first time.
In the light of the anticipated impact of the data from these three trials on clinical practice, the European Association of Percutaneous Coronary Interventions (EAPCI) sought to assess the opinions of the scientific community concerning DAPT duration both before and after AHA 2014. To do this, the association undertook a voluntary web-based survey of the community regarding opinions on DAPT duration after coronary stenting. The current manuscript is a summary of the results.
Methods
This survey initiative was designed to address three major domains concerning DAPT duration: i) clinical practice regarding DAPT duration based on the evidence available before AHA 2014; ii) the expectations of and the reactions to the results of DAPT7, ISAR-SAFE8 and ITALIC9, whose primary findings were presented for the first time during AHA 2014; and iii) the anticipated impact of this new evidence on clinical practice according to the opinion of practising physicians. Accordingly, this survey was built into two sets of questions, distributed before and after the AHA 2014 congress.
The questions included were drafted by the EAPCI Scientific Document Committee and subsequently approved by the EAPCI board. The survey was undertaken using a free web-based survey tool (SurveyMonkey, Palo Alto, CA, USA) and comprised multiple choice questions, including the possibility of adding further comments if required. It was not mandatory to reply to the entire survey. The sample population comprised the mailing list of EuroIntervention – the official journal of the EAPCI. Overall, a total of 15,200 individuals were invited to participate. The invitation to the first part of the survey was sent on the 30th October 2014 and a reminder was sent on the 7th November 2014. For the second part of the survey, the invitation was sent on the 2nd February 2015 and a reminder on the 9th February 2015.
Results
RESPONDENT CHARACTERISTICS
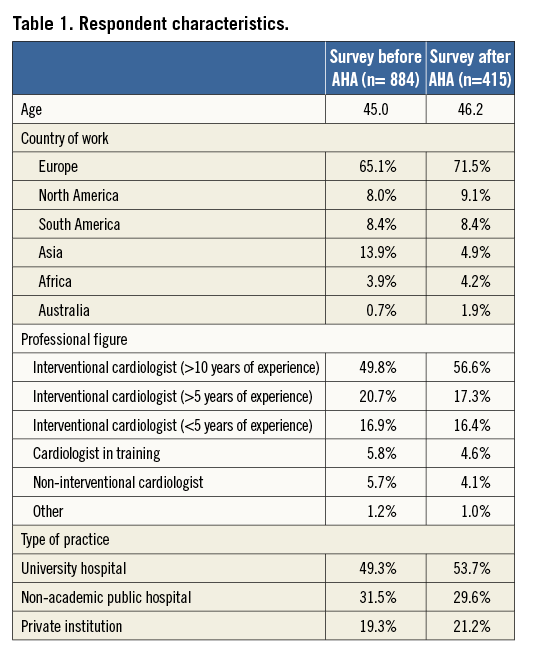
Of the 15,200 invitations sent, a total of 1,134 (7.5%) and 542 (3.6%) physicians responded to the first and the second part of the survey, respectively. Among those, 884 (78%) for the first and 415 (76.6%) for the second part of the survey provided personal and professional information with respect to age, medical and institutional qualification, and geographic region of practice (Online appendix). The characteristics of the respondents are detailed in Table 1. Participation in the survey was global, with the majority of respondents being European (65.1% for the first and 71.5% for the second part of the survey) (Table 1, Online Figure 1). The majority of participants were interventional cardiologists at various career stages (87.4% and 90.3%, respectively), followed by cardiologists in training (5.8% and 4.6%, respectively) and non-interventional cardiologists (5.7% and 4.1%, respectively). A minority of responders declared professional qualifications other than cardiological ones (1.2% and 1%, respectively) (Table 1). About half of participants worked in an academic environment, while the remaining 50% were affiliated to non-university-based centres or private institutions (Table 1). The mean age of respondents was 45 years.
DECLARED CLINICAL PRACTICE OF RESPONDENTS CONCERNING DAPT DURATION BEFORE AHA 2014
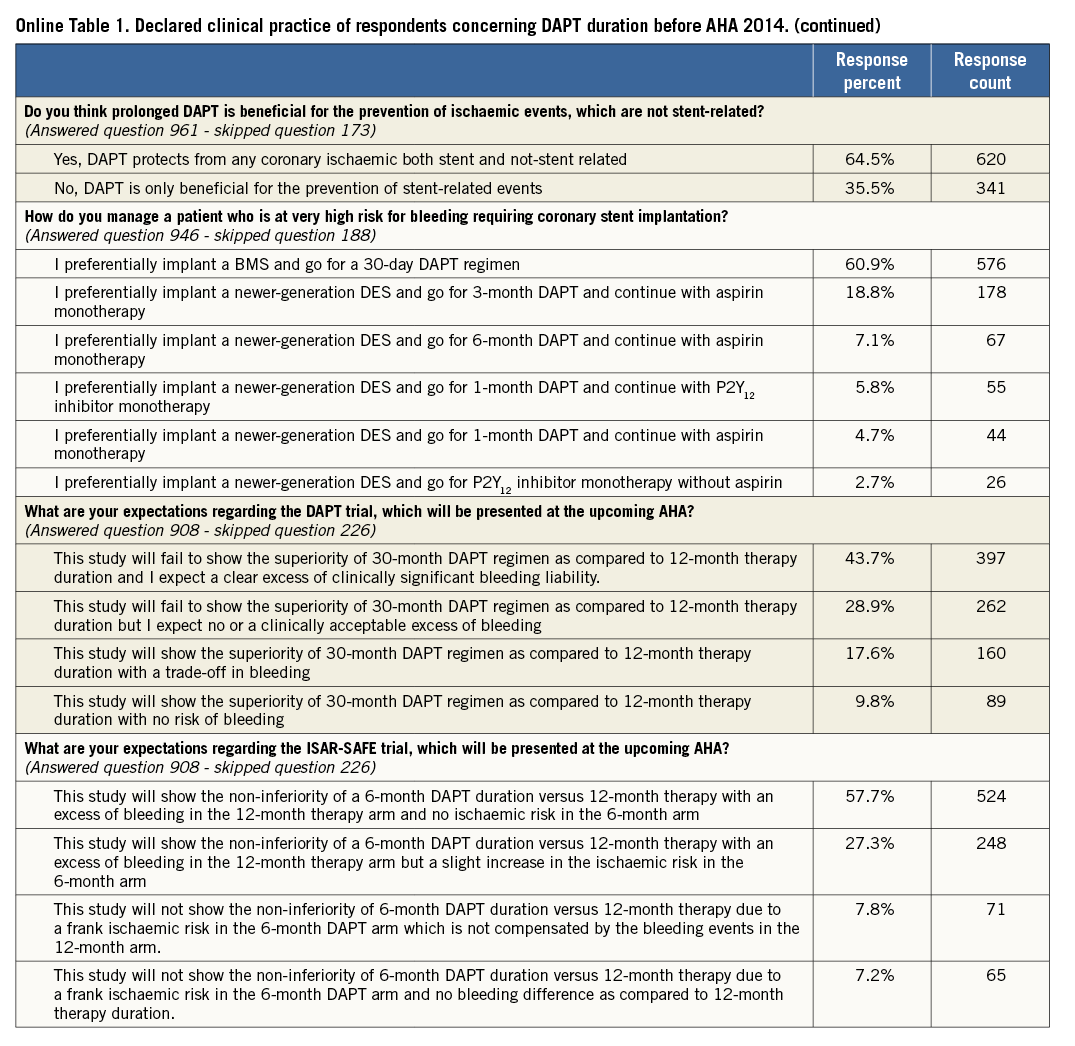
The main findings of this part of the survey are shown in Online Table 1. The majority (53.2%) of respondents indicated a recommendation for a 12-month DAPT duration in all patients treated with drug-eluting stents (DES); one quarter (23.5%) selected a six-month regimen in patients presenting with stable disease and a 12-month regimen for ACS patients; 10.3% routinely prolonged DAPT beyond one year. Three quarters of respondents declared that they take both ischaemic and bleeding risk into consideration when prescribing DAPT. History of stent thrombosis (86%), stenting of the left main or proximal left anterior descending coronary artery (79.7%) and stable versus unstable presentation (74.8%) were the covariates most frequently used in practice to weigh the ischaemic risk (Figure 1). On the other hand, previous bleeding (82.5%), age (76.4%) and renal function (65.3%) have more frequently been identified as important to forecast bleeding (Figure 2). This clinical and/or angiographic set of key covariates used to predict ischaemic or bleeding risk was consistent across institution characteristics (i.e., academic or not academic) and medical qualification/experience (i.e., interventional cardiologist with more than 10 years of experience vs. others, or cardiologist in training vs. others).
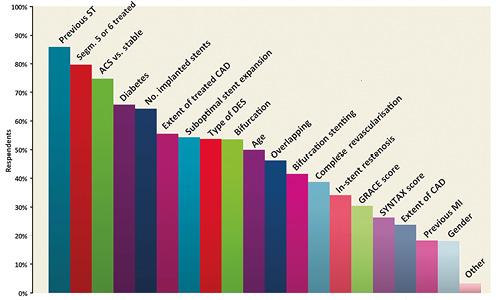
Figure 1. Please select which of the following variables or scores you generally use to weigh the ischaemic risk after DES implantation (multiple answers allowed).
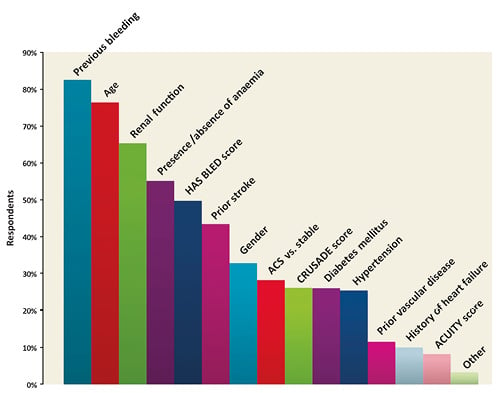
Figure 2. Please select which of the following variables or scores you generally use to weigh the bleeding risk after DES implantation (multiple answers allowed).
With respect to changes to the initially prescribed treatment, 36% of participants reported weighing the occurrence of minor or nuisance bleeding while on DAPT in the decision making on DAPT duration after its prescription, whereas the majority declared adhering to the originally prescribed DAPT duration.
The belief that first-generation DES are more thrombogenic than newer-generation devices and as such require long-term DAPT was widely held (93.5%). However, 54.8% of participants thought that there are still insufficient data to conclude that vulnerability to short DAPT is stent-specific within the class of newer-generation DES. The majority agreed that six-month DAPT is a safe pharmacological strategy after implantation of newer-generation DES, but expressed a need for more clinical data, particularly if a duration shorter than six months is to be recommended, for example after implantation of new-generation non-polymeric DES. The majority also stated that there are insufficient data to draw conclusions on the optimal DAPT duration regimen after bioresorbable everolimus vascular scaffold implantation.
Respondents generally agreed that long-term DAPT exerts protective effects well beyond the prevention of stent-related ischaemic recurrences.
In patients deemed at high risk of bleeding, six responders out of ten (with a gradient noted across professional activity, 75% non-interventional cardiologists and 55% cardiologists in training) would prefer to implant bare metal stents followed by 30-day DAPT.
ANTICIPATION AND INTERPRETATION OF TRIAL RESULTS PRESENTED AT AHA 2014
Before AHA 2014, 41.4% of respondents believed that the evidence guiding DAPT duration in patients receiving DES was average, and 22.8% asserted that it was confusing. The expectations for the upcoming trials were aligned to the results of previous randomised studies available at that time. Indeed, 72.6% expected the DAPT trial not to show the superiority of 30-month vs. 12-month DAPT and 85% expected ISAR-SAFE to show non-inferiority of a six-month DAPT strategy as compared to a 12-month strategy (Online Table 1).
In relation to the DAPT trial, following AHA 2014, 48.5% of respondents interpreted the results of the trial as showing substantial remaining equipoise between the two treatment strategies (i.e., extended duration [30 months] vs. standard duration [12 months]) in terms of efficacy and safety. Against this, 28.4% responded that a standard 12-month DAPT duration remained the preferred clinical strategy (Figure 3), 23.1% reported that that they were convinced of the superiority of 30-month DAPT duration, and 6.1% believed that it should become the new standard of care. These results were consistent across geographic regions. The reasons reported for not adopting the extended duration used in the DAPT trial as a new standard of care were: concern regarding bleeding risk for 75.4% of respondents, the use of a high proportion of early-generation DES in the trial for 55.4% of respondents, concern about the higher mortality observed in the 30-month group for 41.6% of respondents, limited use of new P2Y12 inhibitors for 29.1% of respondents, and the highly selected patient population for 34.2% of respondents, and/or concerns regarding the reproducibility of these results in clinical practice outside trials for 24.6% of respondents (Figure 4).
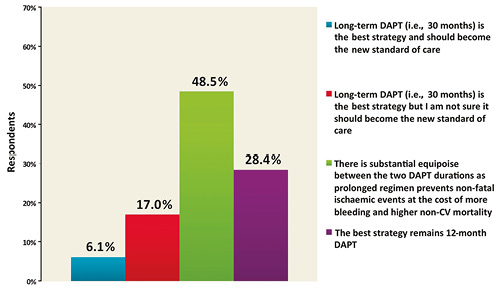
Figure 3. What is your interpretation of the results of the DAPT trial which were presented at AHA and simultaneously published in the New England Journal of Medicine?
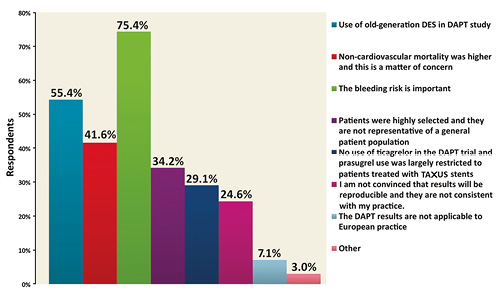
Figure 4. What is/are the reason(s) behind your belief that 30-month DAPT should not become the new standard of care after DAPT trial (multiple answers allowed).
The excess of non-cardiovascular mortality observed in the extended duration treatment arm of the DAPT trial was interpreted as a finding which raises concerns by 32.2% of respondents, while 33.8% would like to know more about this issue (Figure 5). The benefit in terms of reduction of stent thrombosis was related to first-generation DES use in the view of 35% of the respondents, while 30.6% thought that it was not applicable to current practice with new-generation DES, whereas 23.8% thought that this benefit applied to all stent types (Figure 6).
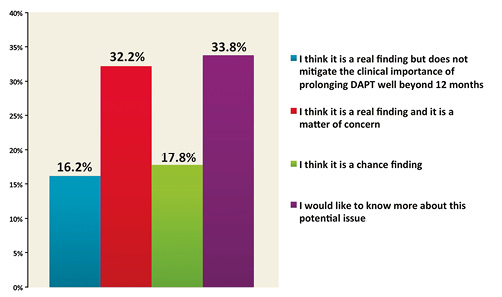
Figure 5. What is your interpretation of the mortality findings in the DAPT trial (i.e., excess of non-cardiovascular mortality in the 30-month DAPT group)?
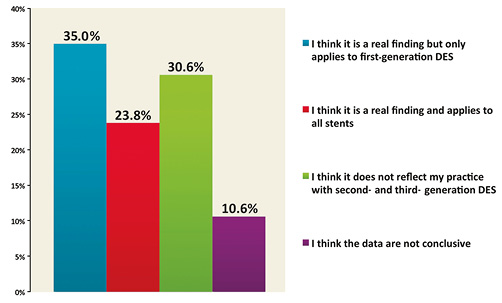
Figure 6. What is your interpretation of the stent thrombosis findings in the DAPT trial (i.e., lower risk of ST with prolonged DAPT irrespective of stent type)?
Evaluating the results of all three studies presented during AHA 2014 in aggregate, 44.4% of respondents believed that the results were compatible with both the possible benefit of long-term DAPT and also the feasibility of stopping therapy early if needed (Figure 7); 22.7% of respondents did not declare a clear opinion and 20.1% found the results contradictory and/or confusing.
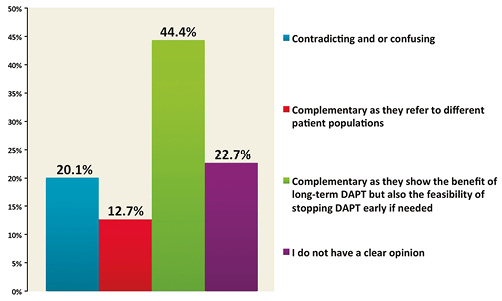
Figure 7. How do you find the results of the DAPT trial as compared to the ISAR-SAFE and ITALIC/ITALIC+ trials?
PRACTICE AFTER THE DAPT, ISAR-SAFE AND ITALIC TRIALS
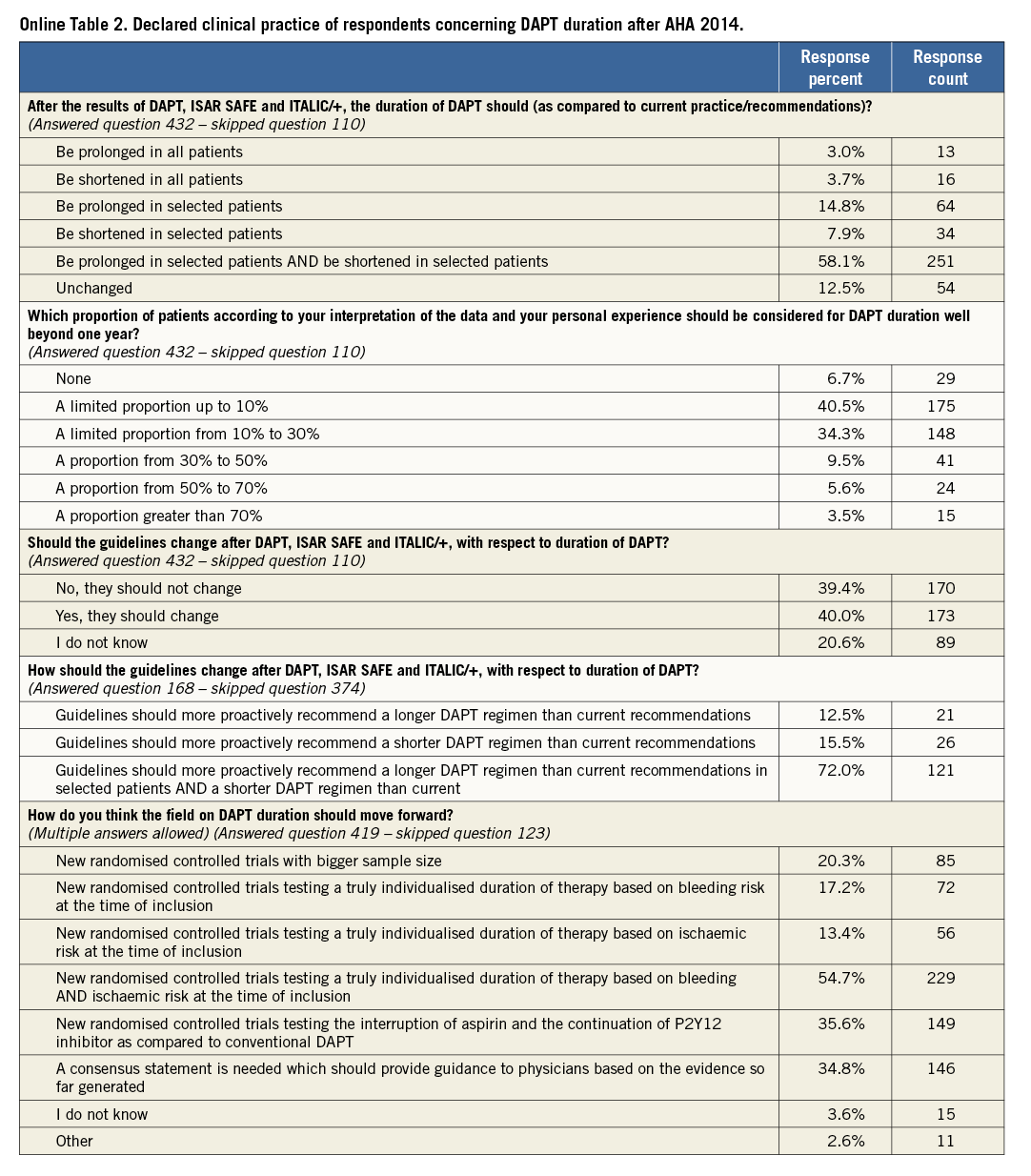
The main findings of this part of the survey are shown in Online Table 2. The majority of respondents (58.1%) indicated that DAPT duration should be individualised, i.e., prolonged in selected patients and shortened in selected patients, as opposed to a 12-month DAPT regimen in all, whereas 12.5% believed that practice and recommendations should not change after the new evidence provided at AHA 2014. Forty percent of respondents believed that a prolonged therapy, beyond one year, should be limited to less than 10% of the patient population; whereas 34% of respondents would treat 10 to 30% of their patients with this strategy (Online Table 2).
Comparing the answers to the parts of the survey delivered before and after AHA, a uniform 12-month DAPT duration in all patients was less frequently selected after AHA 2014 (37.3% before vs. 22.9% after).
The most frequently preferred therapeutic options were: 1) six-month DAPT in stable and 12-month DAPT in ACS patients (24.8% before AHA vs. 29.4% after AHA), 2) DAPT beyond one year in a sizeable proportion of patients (7.4% before AHA vs. 13.0% after AHA), 3) a tailored DAPT duration for individual patients based on ischaemic and/or bleeding risk (9.7% before AHA vs. 16.2% after AHA) (Figure 8). After AHA 2014, the evidence that prolonged DAPT protects against non-stent-related events (64.5% before AHA vs. 71.8% after AHA) was regarded as more compelling than before (Figure 9).
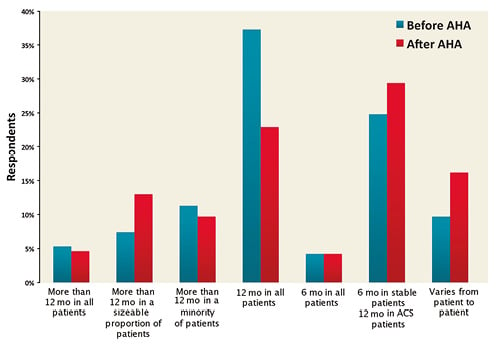
Figure 8. Comparison of the answers to the question “For how long do you generally prescribe DAPT after DES implantation in patients not requiring oral anticoagulation?” before and after AHA.
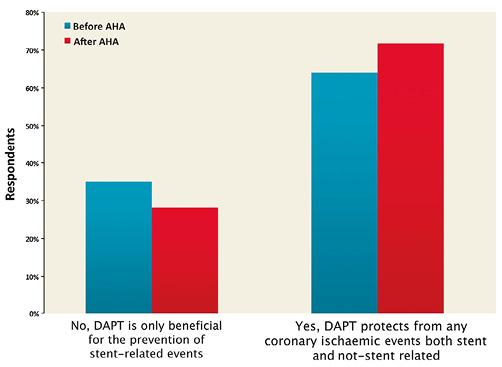
Figure 9. Comparison of the answers to the question “Do you think prolonged DAPT is beneficial for the prevention of ischaemic events, which are not stent-related?” before and after AHA.
In contrast with the opinions expressed before AHA 2014, after the meeting the quality of evidence on DAPT duration in DES recipients was interpreted as “average” by 27.4% of the respondents (as compared to 41.4% of responders before AHA), whereas the majority regarded it as confusing (22.8% before AHA vs. 46.1% after AHA) (Figure 10).
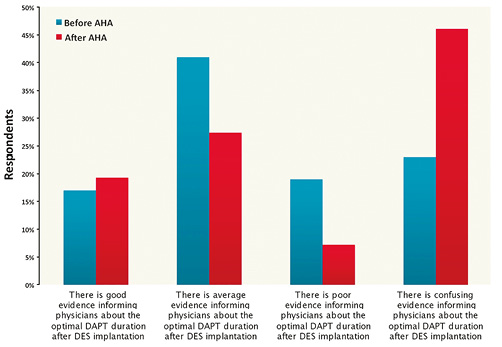
Figure 10. Comparison of the answers to the question “How do you judge the evidence regarding DAPT duration after DES implantation?” before and after AHA.
Overall, 40% of participants called for a change in the guidelines regarding DAPT duration (Online Table 2): the majority of cardiologists working in an academic environment responded in support of a formal change in guidelines supporting practice around DAPT duration, whereas the opposite was voiced by the majority of non-academic cardiologists. When asked about how guidelines should change based on the new evidence, 72% of respondents thought that guidelines should more proactively recommend an individualised therapy in different patient populations (Online Table 2).
Finally, 54.7% of participants believed that new randomised trials testing individualised therapy duration based on ischaemic and bleeding risk are needed, 35.6% expressed the need for trials comparing conventional DAPT versus a P2Y12 inhibitor alone long-term treatment strategy, whereas 34.8% solicited a consensus statement based on the evidence available (Online Table 2). The “other” option was selected by a few calling for new “real-world” prospective registries (two respondents), new randomised trials including potent P2Y12 inhibitors (two respondents), new-generation DES (one respondent) or the implementation of intravascular imaging in decision making (one respondent).
INTERPRETATION OF THE SURVEY RESULTS
The main findings of the EAPCI survey on DAPT duration can be summarised as follows:
– Before AHA 2014, the practice most commonly recommended was 12-month DAPT duration after DES implantation, whereas only one responder out of ten declared a clinical practice consistent with routine DAPT duration beyond one year after stent implantation.
– After AHA 2014, most respondents did not report extended DAPT duration of up to 30 months as representing the preferred approach in comparison with a 12-month treatment duration, and fewer than two responders out of ten believed that this should become the new standard of care.
– After AHA 2014, the evidence regarding DAPT duration was more frequently interpreted as confusing.
– The majority of respondents reported that DAPT should be prolonged or shortened in selected patients according to both ischaemic and bleeding risks and that future guidelines should more proactively recommend strategies in this direction.
– The results of the survey indicate that following the data presented at AHA 2014 considerable confusion exists regarding the optimal duration of DAPT after coronary stenting. The community needs guidance on how DAPT should be individualised and this largely reflects the lack of coordination across DAPT studies performed so far. Many meta-analyses on this topic already exist based on aggregate data, reaching inconsistent conclusions depending on different study selection and methods of analysis. Hence, a collaborative effort among all principal investigators of DAPT studies would be desirable to characterise further the included patient population in each of these and to be able to identify the patients who would most benefit from prolonged versus shortened DAPT and vice versa.
Limitations
This survey has a number of important limitations which should be carefully weighed when interpreting the results. Firstly, only a small percentage of invited practitioners took part in this survey. Therefore, the results are not necessarily representative of the opinion of the whole community. However, low participation rate is a common limitation of surveys in general, especially when the population targeted is that of professionals at an advanced career stage. Secondly, the use of multiple choice questions may lead to question bias. To reduce this effect, respondents were able to add open answers if they felt it was appropriate. In addition, respondents may have been subject to social desirability response bias: for example, this may have overestimated the percentage of those who declared weighing ischaemic and bleeding risks before selecting DAPT duration. Thirdly, the comparison of questions dispensed before and after AHA 2014 was not performed on an individual but on an aggregate basis. As such, it is not possible to evaluate if the single respondent changed his/her opinion or if a new cohort of respondents drove the change in the second part of the survey. However, in view of the relatively high number of contributors, it is likely that we have captured real changes in opinion due to the new evidence provided. Fourthly, this survey was designed and administered before the publication of the results of the PEGASUS trial10, which explored the effects of a prolonged therapy with ticagrelor in patients with previous myocardial infarction. It is possible that the opinion of the respondents may have changed in the light of this new evidence. Finally, the focus of this survey was on duration and not on type of DAPT (i.e., based on which P2Y12 inhibitor). A further EAPCI survey addressing the evidence provided by the PEGASUS study and whether the medical community believes duration of DAPT also to be dependent on type of P2Y12 inhibitor is in preparation.
Conclusions
This EAPCI survey highlights considerable uncertainty within the medical community with regard to the optimal duration of DAPT after coronary stenting in the light of recently reported trial results. The medical community surveyed called for new evidence or updated guidance on how DAPT duration should be individualised for each patient.
| Impact on daily practice Against the conduct of ten dedicated randomised studies investigating various durations of dual antiplatelet therapy (DAPT) and the recent publication of the DAPT trial, which enrolled almost 9,500 patients, the optimal duration of dual antiplatelet therapy after coronary stenting remains unclear. This survey highlights uncertainties within the medical community with regard to how DAPT duration should be managed in clinical practice. A joint effort of international societies, leveraging on the contribution of each principal investigator of the available trials to provide outcomes in pre-specified patient subsets, or ideally the performance of an individual patient meta-analysis, may clarify the most suited DAPT duration for each single patient in practice in future. Providing guidance to the clinical community with respect to the individualisation of the antiplatelet therapy based on patients ischaemic and bleeding risk will be crucial to optimise benefits versus risks. |
Acknowledgements
The authors would like to express their gratitude to the staff of Europa Organisation/EuroPCR for the support given during survey planning and conduct.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix List of respondents
FIRST PART OF THE SURVEY
Aaroe J., Denmark
Aasa M., Sweden
Abdel-Salam A.M., Egypt
Abdulwahab H., Kuwait
Accardi R., Italy
Adel A., Belgium
Al Mowafy A., Kuwait
Al-Najjar Y., United Kingdom
Alaarag A.F., Egypt
Aladashvili A., Georgia
Alawfi K., France
Alcazar De La Torre E., Mexico
Alejos R., Mexico
Alfonso Jimenez V., Spain
Alhashimi H.M.M., Netherlands
Aljeboury A., Iraq
Almeida De Sousa J., Brazil
Almusawi A., Iraq
Alshaikha M., Egypt
Altaf S., Pakistan
Altahmody K.E.A., Egypt
Alvarez Contreras L.R., Mexico
Amarasena N., Sri Lanka
Amoroso G., Netherlands
Anderson R., United Kingdom
Andò G., Italy
Andrade J., Spain
Andreou A.Y., Cyprus
Angulo J., Mexico
Antonio T., Italy
Aprigliano G., Italy
Aquilina M., Italy
Arafa S.E.O., Qatar
Aramberry L., Argentina
Arampatzis C.A., Greece
Araujo J. J., Portugal
Asher E., Israel
Ates I., Turkey
Athanasias D., Greece
Auer J., Austria
Auffret V., France
Ayala F.J., Chile
Baba C., Romania
Baglioni P., Argentina
Bagur R., Canada
Balam-Ortiz E, Mexico
Balducelli M, Italy
Bam Pas G, Greece
Barbash I.M., Israel
Barbosa A. H. P., Brazil
Barbosa R., Brazil
Barnay P., France
Barroso L., Brazil
Basti A., Switzerland
Bax M., Netherlands
Bayet G., France
Beijk M.A., Netherlands
Beltran R., Venezuela
Berenguer Jofresa A., Spain
Berroth R., Germany
Berti S., Italy
Berumen Dominguez L.E., Mexico
Bhasin A., India
Bhaya M., Mauritius
Bianco M., Italy
Biasco L., Denmark
Bikicki M., Serbia
Bonarjee V.V.S., Norway
Bonechi F, Italy
Borges Santos M., Portugal
Boshev M., Macedonia
Bouferrouk A, Algeria
Bounartzidi M., Greece
Bousoula E., Greece
Brie D., Romania
Brtko M., Czech Republic
Brugaletta S., Spain
Brull D.J., United Kingdom
Buchter B, Germany
Buendia R., Philippines
Burzotta F., Italy
Butz T., Germany
Buzzetti F., Italy
Bychowiec B., Poland
Cadeddu M., Italy
Campanile A., Italy
Carneiro J.G., Brazil
Carrilho-Ferreira P., Portugal
Carrillo Guevara J.E., Mexico
Carter A.J., United States
Casal-Heredia H., Venezuela
Castiglioni B., Italy
Castro Fabiano L., Brazil
Cavalcante Silva R., Brazil
Cavalcanti De Oliveira D., Brazil
Cavalcanti R.C., Brazil
Cavazza C., Italy
Centemero M.P., Brazil
Chabane H.K., Italy
Chamié D., Brazil
Chatzis D., Greece
Chaves A.J., Brazil
Cheng S., China
Chinchilla H., Honduras
Ciabatti N., Italy
Cirillo P., Italy
Çitaku H., Albania
Claeys M.J., Belgium
Clifford Cp, United Kingdom
Coceani M., Italy
Cóggiola J., Argentina
Cohen D.J., United States
Conway D.S.G., United Kingdom
Cornelis K., Belgium
Coroleu S. F., Argentina
Corral J.M., Colombia
Cortese B., Italy
Coskun U., Turkey
Costa F., Italy
Costa R.A., Brazil
Coste P., France
Coufal Z., Czech Republic
Cox S., Australia
Cozma A., Romania
Crean P., Ireland
Crenshaw M.H., United States
Cristian U., Romania
Cruz-Alvarado J.E., Mexico
Cuculi F., Switzerland
Cuenza L., Philippines
Cyrne Carvalho H., Portugal
D’Ascenzo F., Italy
D’Urbano M., Italy
Damonte A., Argentina
Dan Florin F, Romania
Dana A., United Kingdom
Dangoisse V, Belgium
De Backer O., Denmark
De Cock D., Belgium
De Vita M., Italy
Debski A., Poland
Delgado A., Mexico
Devadathan S., United Kingdom
Dhamrait S., United Kingdom
Di Lorenzo E., Italy
Di Serafino D., Italy
Diego-Nieto A., Spain
Dievart F., France
Diez J.L., Spain
Dimitriadis K., Greece
Dina C., Romania
Doerner O., Germany
Donahue M., Italy
Donis J., Venezuela
Drieghe B., Belgium
Drissi M.F., Tunisia
Du Fretay H., France
Dziewierz A., Poland
Echavarría-Pinto M., Spain
Echeverria Romero R.G., Honduras
Economou F., Greece
Eftychiou C., Cyprus
Egdell R., United Kingdom
El Hosieny A., Saudi Arabia
El Meguid K., Egypt
Elabbassi W., United Arab Emirates
Elesgerli S., Azerbaijan
Elghetany H., Saudi Arabia
Elizondo J.C., Costa Rica
Elkahlout A., Romania
Elrowiny R., Egypt
Elserafy A.S., Egypt
Emam A., Egypt
Emara A., Egypt
Emmanouil P., Greece
Ercilla J., Peru
Erglis A., Latvia
Eslam Taha E., Egypt
Esmaeil S., Egypt
Esposito G., Italy
Ettori F., Italy
Eugenio N., Brazil
Everaert B., Netherlands
Ezquerra Aguilar W., Peru
Falu R., Argentina
Farag E., Egypt
Farjalla J., Brazil
Feldman L., France
Feldman M., Argentina
Felice H., Malta
Fernandez-Nofrerias E., Spain
Fernández-Rodríguez D., Spain
Ferranti F., Italy
Ferreira Q., Qatar
Ferrone M., Italy
Fleischmann C., Germany
Flessas D., Greece
Formigli D., Italy
Fozilov H., Uzbekistan
Fraccaro C., Italy
Freitas J.O., Brazil
Fresco C., Italy
Fridrich V., Slovakia
Furmaniuk J., Poland
Gagnor A., Italy
Galasso G., Italy
Galeazzi G.L., Italy
Galli S., Italy
Galvez Villacorta V., Peru
Gandolfo C., Italy
García E., Spain
García-Blas S., Spain
Garducci S., Italy
Garg S., United Kingdom
Garro N., Italy
Gatto L., Italy
Georgiou M.G., Cyprus
Ghanem I., Egypt
Ghose T., India
Giacchi G., Italy
Giang P.T., Viet Nam
Giesler T., Germany
Giovino M., Italy
Girardi P., Italy
Girasis C., Greece
Giunio L., Croatia
Giustino G., United States
Glatthor C., Germany
Glogar H.D., Austria
Golledge P., United Kingdom
Gomez Moreno J., Argentina
Gómez Recio M., Spain
Gommeaux A., France
Grantalis G., Greece
Greco F., Italy
Grundeken M.J., Netherlands
Grunert S., Germany
Guðmundsdóttir I., Iceland
Guenoun M., France
Guerios E., Brazil
Gupta R., United Arab Emirates
Gupta S., India
Gutièrrez C., Mexico
Hafeez I., India
Halvorsen S., Norway
Hamed Hussein G.A., Saudi Arabia
Hammoudeh A., Jordan
Hansen P.R., Denmark
Harb S., Austria
Hawas J.M., Iraq
Hayrapetyan H., Armenia
Heintzen M.P., Germany
Hengstenberg C., Germany
Herity N., United Kingdom
Hernandez F., Spain
Heyse A., Belgium
Hicham D., Lebanon
Hildick-Smith D., United Kingdom
Hill J., United Kingdom
Hillani A., France
Hiltrop N., Belgium
Hiramori A., Japan
Hobson A.R., United Kingdom
Homan D.J., United States
Hooda A., India
Ielasi A., Italy
Ierna S., Italy
Iftikhar A.K., Pakistan
Ilic I., Serbia
Imai Y., Japan
Imperadore F., Italy
Indolfi C., Italy
Iorga V., Romania
Ipek E., Turkey
Ito S., Japan
Jacksch R., Germany
Jae-Sik J., South Korea
James S., Sweden
Jamshidi P., Switzerland
Jerbi J., Tunisia
Jimenez Quevedo P., Spain
Jimenez-Navarro M., Spain
Jiménez-Santos M., Mexico
Jin Q.H., China
Joksas V., Lithuania
Jovic D., Serbia
Junejo S., United Kingdom
Kallel R., Tunisia
Kamal A., Egypt
Kamiya H., Japan
Kannan D., India
Kantaria M., Georgia
Kapetanopoulos A., Greece
Kara Ali B., Lebanon
Karjalainen P.P., Finland
Karthikeyan V.J., United Kingdom
Kato R., Japan
Katsikis A., Greece
Kefer J., Belgium
Keta D., Germany
Ketteler T., Germany
Khan M., United Kingdom
Kharlamov A., Russian Federation
Kinani A., Iraq
Kinani T., Iraq
Kinnaird T., United Kingdom
Kislo A., Poland
Kiviniemi T., Finland
Kleiban A., Argentina
Kluck B., United States
Kocayigit I., Turkey
Kokis A., Canada
Komiyama N., Japan
Konstantinos L., Greece
Kordalis A., Greece
Kozak M., United States
Krecki R., Poland
Kristensen S.D., Denmark
Krizanic F., Germany
Krsticevic L., Argentina
Kuex H., Germany
Kukreja N., United Kingdom
Kulić M., Bosnia and Herzegovina
Kulikovskikh Y.V., Russian Federation
Kulkarni P., India
Kumar N., Netherlands
Kumar Soni A., India
Kuzmenko E., Russian Federation
L’Allier P.L., Canada
Langner O., Germany
Lapin O., Russian Federation
Lauer B., Germany
Leclercq F., France
Leibundgut G., Switzerland
León Aliz E., Cuba
Leon C., Venezuela
Leon K., Egypt
Leoncini M., Italy
Leone A.M., Italy
Leroux L., France
Lesiak M., Poland
Letilovic T., Croatia
Lev E., Israel
Linares Vicente J.A., Spain
Lindsay S., United Kingdom
Loh P.H., Singapore
Loncar G., Serbia
Loo B., Ireland
Lopez M.B., Mexico
Lopez-Cuellar J., Mexico
Lozano I., Spain
Luigia P., Italy
Lunde K., Norway
Lyczywek M., Poland
Macdougall D., United Kingdom
Mafrici A., Italy
Magni V., Italy
Magro M., Netherlands
Mainar V., Spain
Makarović Z., Croatia
Malik N., United Kingdom
Maly M., Czech Republic
Mansour S., Canada
Marenco R.E., Honduras
Maresta A., Italy
Marinho G.E., Brazil
Marino R.L., Brazil
Marinucci L., Italy
Martins H., Brazil
Martins J., United Kingdom
Mashayekhi K., Germany
Masood A., Pakistan
Maurer E., Austria
Mavrogianni A.D., Greece
Mazurek T., Poland
Medina A., Mexico
Mehilli J., Germany
Mellwig K.P., Germany
Mendez M., Chile
Mendiz O.A., Argentina
Meneses A., Mexico
Mercado L.A., Bolivia
Mereuta A., Romania
Mezzapelle G., Italy
Milanovic N., Bosnia and Herzegovina
Mohamed S.M., Egypt
Mohanad A., Egypt
Mohanty A., India
Moorthy N., India
Morales F.J., Spain
More R., United Kingdom
Moreno Samos J.C., Spain
Moreno-Martínez F.L., Spain
Moscato F., Italy
Mossmann M., Brazil
Mrevlje B., Germany
Müller-Eichelberg A., Germany
Muraglia S., Italy
Musumeci G., Italy
Nadir Khan M., Pakistan
Najim S., United Kingdom
Nakamura S., Japan
Nakao F., Japan
Näveri H., Finland
Negus B., United States
Nerla R., Italy
Nguyen H.T., United States
Niess G.S., United States
Nikas D.N., Greece
Niroomand F., Germany
Niva J., Finland
Nogueira J.W., Brazil
Nombela-Franco L., Spain
Notrica M., Argentina
Nouri B., Tunisia
Nugue O., France
Nunes G.L., Brazil
Ober M., Romania
Ochoa J., Colombia
Oh J.H., South Korea
Ojeda S., Spain
Oktay Tureli H., Turkey
Olowe Y., United States
Oluseun A., United States
Opolski G., Poland
Ornelas C.E., Brazil
Otasevic P., Serbia
Ozturk A., Turkey
Padilla F., Mexico
Pagny J.Y., France
Paolantonio D., Argentina
Papaioannou G.I., Greece
Parodi G., Italy
Patil S.N., India
Pavei A., Italy
Pavìa A., Mexico
Pavlidis A., United Kingdom
Pell A., United Kingdom
Percoco G.F., Italy
Pernasetti L.V., Spain
Pescoller F., Italy
Petropoulakis P., Greece
Piatti L., Italy
Picardi E., Italy
Pieroni D.M., Argentina
Pina J., United States
Pinheiro L.F., Brazil
Pinto F.J., Portugal
Pipa J.L., Portugal
Piroth Z., Hungary
Pisano F., Italy
Podbregar M., Slovenia
Polak G., Poland
Polimeni A., Italy
Postadzhiyan A., Bulgaria
Postu M., Romania
Poulimenos L.E., Greece
Pow Chon Long F., Ecuador
Poyet R., France
Pradhan Ak, India
Predescu L.M., Romania
Prida X.E., United States
Prof. Aly Saad, Egypt
Prog R., Germany
Pulikal D.G.A, United Kingdom
Qiangzhong P.I., China
Radu M.D., Denmark
Rajendran D., India
Ram Anil Raj M.R., India
Ramazzotti V., Italy
Rapacciuolo A., Italy
Ratib K., United Kingdom
Raungaard B., Denmark
Raviola E., Italy
Reppas E., Greece
Reyes J.A., Dominican Republic
Rezek M., Czech Republic
Riess G.J., Germany
Rifaie O., Egypt
Rigattieri S., Italy
Rissanen T., Finland
Ristic A.D., Serbia
Rittger H., Germany
Roberts J., United States
Rodríguez Saavedra A., Argentina
Roik M., Poland
Roshan Rao K, India
Routledge H., United Kingdom
Rubboli A., Italy
Rudolph T., Germany
Rudzitis A., Latvia
Ruiters Aw, Netherlands
Ruiz Ros J.A., Spain
Ruiz-García J., Spain
Ruiz-Nodar J.M., Spain
Sabate M., Spain
Sabnis G., India
Sabouret P., France
Sacra C., Italy
Saghatelyan M., Armenia
Sahin M., Turkey
Said S., Netherlands
Salachas A., Greece
Salas Llamas J.P., Mexico
Salih A., Saudi Arabia
Sanchez O.D., United States
Sánchez-Gila J., Spain
Sanchez-Perez I., Spain
Santarelli A., Italy
Sardovski, Bulgaria
Sarenac D., Serbia
Sarma J., United Kingdom
Sarno G., Sweden
Savonitto S., Italy
Sayied Abdullah A., Ireland
Schäfer A., Germany
Scherillo M., Italy
Schneider H., Germany
Schühlen H., Germany
Sciahbasi A., Italy
Seca L., Portugal
Sedlon P., Czech Republic
Semenka J., Czech Republic
Serra L.A., Spain
Sesana M., Italy
Sethi A., United Kingdom
Sgueglia G.A., Italy
Shaheen S., Egypt
Shahri H., Iran
Sheiban I., Italy
Shyu K.G., Taiwan
Silva C.E., Brazil
Sionis D., Greece
Siqueira D.A., Brazil
Siqueira M.J., Brazil
Smits P., Netherlands
Sobhy M., Egypt
Sokolov M., Ukraine
Soliman S., Egypt
Somani A.N., India
Sridhar G., Malaysia
Stakos D., Greece
Šťásek J., Czech Republic
Stefanini G., Switzerland
Steigen T.K., Norway
Stewart, New Zealand
Stipal R., Czech Republic
Stochino M.L., Italy
Stoel M.G., Netherlands
Stoyanov N., Bulgaria
Subla R.M., United States
Suliman A., Sudan
Summaria F., Italy
Syarif R., Indonesia
Syed A.A., United States
Tanaka Y., Japan
Tashani A., Libya
Tauzin S., France
Tawade N., India
Tawfik M., Egypt
Tayeh O., Egypt
Terzic I., Bosnia Herzegovina
Testa L., Italy
Thevan B., Bahrain
Thiam M., Senegal
Tiecco F., Italy
Tierala I., Finland
Tilea I., Romania
Tilsted H. H., Denmark
Tomasik A.R., Poland
Tonev I., Bulgaria
Torres Bosco A., Spain
Tousek P., Czech Republic
Townend J., United Kingdom
Tran Ngoc T., Viet Nam
Triantafyllou K., Greece
Tsigkas G., Greece
Tsioufis C., Greece
Turri M., Italy
Tyligadis G., Greece
Ugo F., Italy
Ultramari F.T., Brazil
Urban P., Switzerland
Uren N., United Kingdom
Uretsky B.F., United States
Uribe C.E., Colombia
Usman B., Kazakhstan
Valadez Molina F., Mexico
Van Houwelingen K.G., Netherlands
Vandormael M., United States
Varvarovsky I., Czech Republic
Vassilis V., Greece
Velasquez D., Colombia
Verdoia M., Italy
Vermeersch P., Belgium
Vidal-Perez R., Spain
Vinesh J., India
Violini R., Italy
Vista J.H., Mexico
Vogt F., Germany
Vogt M., Germany
Vokac D., Slovenia
Vom Dahl J., Germany
Vranckx P., Belgium
Wahab A., India
Wang R., Brazil
Wang T.D., Taiwan
Wani S., India
Weisz S.H., Italy
Werner G.S., Germany
Wilkinson J.R., United Kingdom
Wolf A., Germany
Youssef A., Egypt
Yumoto K., Japan
Zaderenko N., Argentina
Zaghloul Darwish A., Egypt
Zahn R., Germany
Zaro T., Italy
Zavalloni D., Italy
Zbinden R., Switzerland
Zekanovic D., Croatia
Zhang B., China
Zhang C., China
Zhang Y.J., China
Zhonghan N., China
Zingarelli A., Italy
Zueco J., Spain
Zuhairy H., Ireland
SECOND PART OF THE SURVEY
Aaroe J., Denmark
Abbate A., United States
Abdel Hamid M., Egypt
Abdelmegid M.A.F., Egypt
Acuña-Valerio J., Mexico
Adriaenssens T., Belgium
Agostoni P., Netherlands
Aikot H., India
Alameda M., Spain
Alcaraz H., Mexico
Almendro-Delia M., Spain
Altug Cakmak H., Turkey
Amir A., United Kingdom
Amoroso G., Netherlands
Andò G., Italy
Andrade J., Spain
Arampatzis C.A., Greece
Arjomand A., Australia
Assomull R., United Kingdom
Atalar E., Turkey
Auer J., Austria
Auffret V., France
Avramides D., Greece
Aytek Şimşek M., Turkey
Aznaouridis K., United Kingdom
Azpeitia Y., Mexico
Baglioni P., Spain
Barnabas C., South Africa
Barsness G.W., United States
Bartorelli A.L., Italy
Basoglu A., Belgium
Bayet G., France
Benezet J., Spain
Benincasa S., Italy
Berland J., France
Berrocal D.H., Argentina
Berroth R., Germany
Bett N., Australia
Bhaya M., Mauritius
Bianco M., Italy
Boskovic S., Serbia
Brandão V., Portugal
Brtko M., Czech Republic
Brull D.J., United Kingdom
Buchter B., Germany
Caporale R., Italy
Caprotta F., Argentina
Carrabba N., Italy
Carrilho-Ferreira P., Portugal
Casal-Heredia H., Venezuela
Cazaux P., France
Chaves A.J., Brazil
Cheniti G., Tunisia
Chinchilla Calix H., Honduras
Chung W.Y., South Korea
Cicco N., Germany
Cicco N.A., Germany
Cieza T., Canada
Clapp B., United Kingdom
Coceani M., Italy
Commeau P., France
Conway D., United Kingdom
Cortese B., Italy
Cuellar C., Colombia
D’Urbano M., Italy
Damonte A., Argentina
De Backer O., Denmark
De Benedictis M., Italy
De La Torre Hernandez J.M., Spain
De Vroey F., Belgium
Degertekin M., Turkey
Di Lorenzo E., Italy
Diez J.L., Spain
Dina C., Romania
Eberli F.R., Switzerland
Echavarria-Pinto M., Spain
Eggebrecht H., Germany
Ekicibasi E., Turkey
Elmaraghi M., Egypt
Előd P., Hungary
Ercilla J., Peru
Ergene A.O., Turkey
Ezquerra Aguilar W., Peru
Fadlalla V.F., Egypt
Farah M.A., Argentina
Fernandez Viña R., Argentina
Fernández-Rodríguez D., Spain
Ferro A., Italy
Fischer D., Germany
Floré V., Belgium
Foley D.P., Ireland
Formigli D., Italy
Fresco C., Italy
Furmaniuk J., Poland
Gafoor S., Germany
Gallo S., Paraguay
Garg S., United Kingdom
Gaspardone A., Italy
Gavrilescu D., Romania
Gentiletti A., Argentina
Giacchi G., Spain
Gilard M., France
Giovannelli F., Italy
Glogar H.D., Austria
Gomez Moreno J.O., Argentina
Gomez Recio M., Spain
Gommeaux A., France
Gonzalez Pacheco I., Mexico
Gonzalo N., Spain
Grajek S., Poland
Greco F., Italy
Gurgel De Medeiros J.P., Brazil
Haine S., Belgium
Hakim D., United States
Hakim Vista J.J., Mexico
Hallani H., Australia
Hamid M., Sweden
Hansen P.R., Denmark
Heintzen M.P., Germany
Helft G., France
Heppell R.M., United Kingdom
Hernández-Enríquez M., Spain
Hlinomaz O., Czech Republic
Ho Choo E., South Korea
Huqi A., Italy
Hurtado E.O., Colombia
Iakovou I., Greece
Ielasi A., Italy
Imperadore F., Italy
Iosseliani D., Russian Federation
Ipek E., Turkey
Jacksch R., Germany
Janssens L., Belgium
Jean M., France
Jensen J.K., Denmark
Jensen, J., Sweden
Jesudason P., Malaysia
Jimenez Diaz V.A., Spain
Karchevsky D., Russian Federation
Karpovskii A., Russian Federation
Katsimagklis G., Greece
Kereiakes D., United States
Kersanova N.C., Chile
Kesavan S., United Kingdom
Ketteler T., Germany
Khaled H., Egypt
Khalil S.A, Morocco
Khan M., United Kingdom
Kharlamov A., Russian Federation
Kiatchoosakun S., Thailand
Kim K.S., South Korea
Kinnaird T., United Kingdom
Kirma C., Turkey
Kleiban A., Argentina
Koltowski L., Poland
Konteva M., Bulgaria
Kozinski L., Poland
Kuehn C.R., Germany
Kukreja N., United Kingdom
Kulić M., Bosnia and Herzegovina
Kumar S., United States
Kyriakakis C.G., South Africa
Laanmets P., Estonia
Labrunie A., Brazil
Ladwiniec A., United Kingdom
Lai G., Italy
Laine M., France
Latib A., Italy
Lattuca B., France
Lauer B., Germany
Lazarevic A.M., Bosnia and Herzegovina
Lee K.S., United States
Legrand V., Belgium
Leiva G., Argentina
Leoncini M., Italy
Leoncini M., Italy
Lester N., South Africa
Letilovic T., Croatia
Levchyshyna O., Ukraine
Linares Vicente J.A., Spain
Livia G., Spain
Londero H.F., Argentina
Luha O., Austria
Lunde K., Norway
Lupi A., Italy
Lupkovics G., Hungary
Maaliki S., United States
Macdougall D., United Kingdom
Maeng M., Denmark
Mahr N.C., United States
Mansour S., Canada
Mantyla P., Finland
Mariano E., Italy
Marsit N., Tunisia
Martins H.C., Brazil
Mcdonough T.J., United States
Medda M., Italy
Mejia Viana S., Spain
Mendiz O.A., Argentina
Merigo Azpir C.A., Mexico
Mezzapelle G., Italy
Milanovic N., Bosnia and Herzegovina
Mitreski S., Macedonia
More R., United Kingdom
Moreno R., Spain
Moreu J., Spain
Moscato F., Italy
Muehler M., Germany
Muir D., United Kingdom
Munoz Molina R., Guatemala
Musilli N., Italy
Myć J., Poland
Nadra I., Canada
Nagy C.D., United States
Narayanan A., United States
Neugebauer P., Slovakia
Nguyen M, Canada
Nick H., Belgium
Nicolino A., Italy
Obradovic S.D., Serbia
Padilla F., Mexico
Paizis I., Greece
Panagiotis P., Greece
Park S.D., South Korea
Park S.J., South Korea
Pasquetto G., Italy
Patel D., United Kingdom
Paunovic D., Belgium
Pavìa A., Mexico
Pedon L., Italy
Pereira Machado F., Portugal
Pershukov H., Russian Federation
Petrou E., Greece
Piatti L., Italy
Pinheiro L.F., Brazil
Pinton F.A., Brazil
Pisano F., Italy
Polak G., Poland
Poyet R., France
Preti G., Italy
Prog R., Germany
Puri R., Canada
Pyxaras S.A., Germany
Quintanilla J., Mexico
Ramazzotti V., Italy
Raviola E., Italy
Rhouati A., Algeria
Ribeiro De Oliveira I., Brazil
Rigattieri S., Italy
Rissanen T., Finland
Rivetti L., Italy
Rodriguez A.E., Argentina
Rotevatn S., Norway
Routledge H., United Kingdom
Rubartelli P., Italy
Rubboli A., Italy
Ruiz-García J., Spain
Saad A., Egypt
Sabate M., Spain
Sabouret P., France
Sachdeva R., United States
Said S., Netherlands
Salachas A.J., Greece
Sanchez-Perez H., Spain
Sangiorgi G., Italy
Santarelli A., Italy
Santoro G.M., Italy
Saporito F., Italy
Sarenac D., Serbia
Savonitto S., Italy
Scappaticci M., Italy
Schmermund A., Germany
Schmidt J.E., United States
Schmitz T., Germany
Schneider H., Germany
Schneider T.I., Germany
Schuchlenz H., Austria
Schühlen H., Germany
Sepúlveda Varela P., Chile
Sesana M., Italy
Sethi A., United Kingdom
Shaw E., Australia
Silva C.E.F., Brazil
Silva Marques J., Portugal
Sionis D., Greece
Skalidis E., Greece
Slhessarenko J., Brazil
Spauldingc., France
Stakos D., Greece
Stankovic G., Serbia
Stasek J., Czech Republic
Stefanini G.G., Italy
Suwannasom P., Thailand
Synetos A., Greece
Szuster E., Brazil
Taha S., Egypt
Tavano D., Italy
Tebet M., Brazil
Thury A., Hungary
Tilsted H.H., Denmark
Tomasik A., Poland
Toutouzas K., Greece
Triantafyllis A.S., Greece
Tsikaderis D., Greece
Tumscitz C., Italy
Turri M., Italy
Tyligadis G., Greece
Tzanogiorgis I., Greece
Udovichenko A., Russian Federation
Ulrike N., Austria
Unikas R., Lithuania
Uren N., United Kingdom
Uretsky B.F., United States
Valerio M.G., United States
Van Mieghem C., Belgium
Vandendriessche T., Belgium
Vavlukis M., Macedonia
Vidal-Perez R., Spain
Vigna C., Italy
Vilar J.V., Spain
Vizzari G., Italy
Vogt F., Germany
Voudris V., Greece
Wafa S., Egypt
Wagner D.R., Luxembourg
Webster M., New Zealand
Wichter T., Germany
Wiedemann S., Germany
Williams P.D., United Kingdom
Woody W., United States
Yding A., United Kingdom
Zachow G., Germany
Zaderenko N., Argentina
Zaghloul Darwish A.M., Egypt
Zbinden R., Switzerland
Zhang Y.J., China
Zingarelli A., Italy
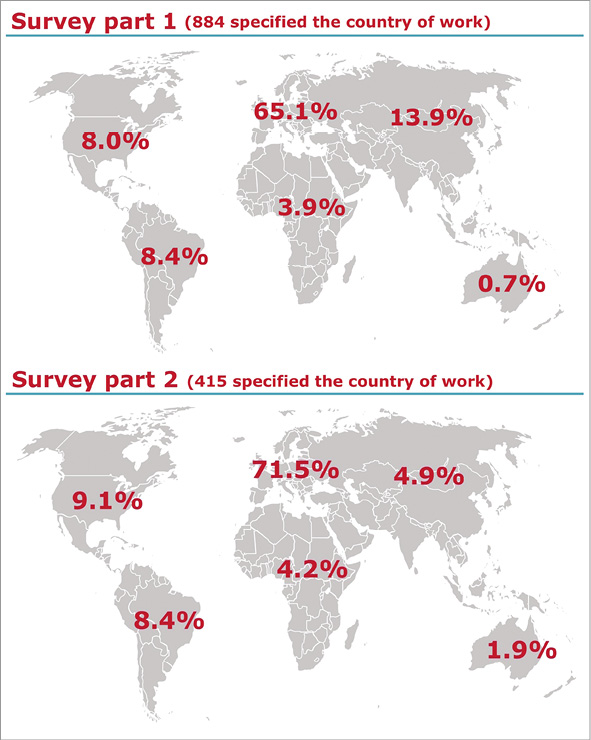
Online Figure 1. Geographic region of practice of the respondents.