
Abstract
Aims: There are few randomised studies concerning the optimal duration of dual antiplatelet therapy (DAPT) for patients who receive a second-generation drug-eluting stent (DES). This trial aimed to investigate the safety of six-month compared with 12-month DAPT maintenance after second-generation DES implantation.
Methods and results: A prospective, randomised, multicentre trial was performed at 10 medical centres. The 1,368 patients included in the study received a biolimus-eluting stent (BES) or a zotarolimus-eluting stent (ZES). The primary outcome measured was the composite of major adverse cardiac events (MACE), including cardiac death, myocardial infarction (MI), or ischaemia-driven target lesion revascularisation at the 12-month follow-up. The secondary outcome was the percentage of uncovered struts at six months in 60 patients (30 ZES, 30 BES) using optical coherence tomography (OCT) assessment. Each patient was randomly assigned to six-month (n=684) or 12-month DAPT (n=684). Major adverse cardiac events at 12 months occurred in eight patients (1.2%) in the six-month DAPT group and in four patients (0.6%) in the 12-month DAPT group (risk difference 0.6%; 95% confidence interval [CI]: -0.4–1.6%; p=0.24). The upper 95% CI limit was lower than the pre-specified limit of 4% non-inferiority (p for non-inferiority <0.05). The percentage of uncovered struts was 3.16±4.30% at six months in 60 stents of 60 patients.
Conclusions: After second-generation DES implantation, six-month DAPT was not inferior to 12-month DAPT in terms of MACE occurrence over the 12-month follow-up period. OCT examination revealed favourable stent strut coverage at six months after stent implantation. The current trial is registered under ClinicalTrials.gov Identifier: NCT03056118.
Abbreviations
BES: biolimus-eluting stent
DAPT: dual antiplatelet therapy
DES: drug-eluting stent(s)
MACE: major adverse cardiac events
OCT: optical coherence tomography
MI: myocardial infarction
TLR: target lesion revascularisation
TVR: target vessel revascularisation
ZES: zotarolimus-eluting stent
Introduction
Stent thrombosis after drug-eluting stent (DES) implantation is strongly associated with early discontinuation of dual antiplatelet therapy (DAPT)1. The risks associated with first-generation DES use have been revealed in previous studies. For example, there is a higher incidence of late stent thrombosis, compared with bare metal stent (BMS) use, which is related to impaired or delayed re-endothelialisation2. Prolonged DAPT (i.e., ≥12 months) has been recommended to prevent late or very late stent thrombosis in first-generation DES patients3.
Vascular imaging studies have revealed that the use of second-generation DES results in improved stent healing and better safety and efficacy, compared with the use of first-generation DES4,5. Clinical trial results indicate that there are no significant differences in clinical outcome in populations after implantation of second-generation DES6-8. Based on these results, current practice guidelines recommend a six-month DAPT duration after second-generation DES implantation for patients with stable coronary artery disease.
However, clinical studies are needed to determine whether these differences increase stent safety and efficacy and whether a ≤12-month DAPT duration, after implantation of BES or ZES, is safe for the patient. The objective of the OPTIMA-C trial (OPTIMAl Duration of Clopidogrel after Implantation of Second-generation Drug-eluting Stents) was to investigate the safety of six-month compared with 12-month DAPT maintenance after second-generation DES implantation.
Methods
STUDY DESIGN
The OPTIMA-C trial was an investigator-initiated, prospective, open-label, randomised, multicentre study with a BES (BioMatrix™; Biosensors International Limited, Singapore) or ZES (Resolute Integrity™; Medtronic, Minneapolis, MN, USA) for treatment of native coronary lesions. The trial was performed at 10 medical centres in South Korea (Supplementary Appendix 1). Our hypothesis was that six-month DAPT would not be inferior to 12-month DAPT in patients who underwent second-generation DES implantation. Study coordination, data management, and site management services were performed at the Cardiovascular Research Center, Seoul, South Korea. At regular intervals, the adverse clinical event data were independently reviewed for accuracy and completeness by a clinical events committee.
STUDY POPULATION
Patients were eligible for enrolment if implantation of a second-generation DES for native coronary lesion (lesion length ≤28 mm, stent length ≤30 mm) treatment was indicated based on angiography results. The inclusion criteria were: patient age ≥20 years, eligible for percutaneous coronary intervention (PCI), implantation with at least one BES or ZES. The excluded clinical conditions were acute ST-elevation myocardial infarction (MI) and treatment of the left main artery, true bifurcation lesions requiring two stents, and target lesions with chronic total occlusion. Detailed inclusion and exclusion criteria are presented in Supplementary Appendix 2. The study protocol was reviewed and approved by the ethics committees at each clinical site before initiation of the study. Written informed consent was obtained from each patient before inclusion in the study population.
RANDOMISATION AND STUDY PROCEDURES
A concealed interactive web-based system was used to assign randomly (1:1 scheme) each study participant to receive six- or 12-month DAPT immediately after a diagnostic angiogram was performed. Either BES or ZES was randomly assigned to minimise bias from different stent devices with a 1:1 ratio. Stratification was based on enrolment site and presence of diabetes mellitus.
PCI was performed using standard angiographic or intravascular imaging guidance protocols. Aspirin (100 mg daily dose, indefinitely) and clopidogrel (75 mg daily dose, >6 months) were prescribed to all patients following stent implantation.
Optical coherence tomography (OCT) assessment was performed at six months after stent implantation in 60 patients (30 BES and 30 ZES) who agreed to participate in an OCT substudy. Target lesion imaging was performed using a frequency-domain OCT system (C7-XR OCT imaging system; LightLab Imaging, Inc./St. Jude Medical, St. Paul, MN, USA). All OCT images were analysed at the core laboratory (Cardiovascular Research Center, Seoul, South Korea) by analysts who were blinded to patient and procedural information. The measurements were performed following previously proposed definitions9,10. The patients who agreed to the follow-up procedure underwent follow-up coronary angiography at 12 months post implantation.
STUDY ENDPOINTS AND FOLLOW-UP
The primary endpoint was a composite of major adverse cardiovascular events (MACE; cardiac death, target vessel-related MI, ischaemia-driven target lesion revascularisation [TLR] at 12 months). Academic Research Consortium guidelines were used to define the clinical events11. The secondary endpoints were the percentage of uncovered stent struts at six months post implantation in a specified group of 60 patients and late luminal loss in the patients who underwent follow-up coronary angiography. Ischaemia-driven TLR was defined as a repeat PCI or bypass surgery of the target lesions11. DAPT score was previously validated, and it was applied to assess the risk of ischaemia and bleeding for each patient12.
Follow-up clinical assessment was performed at 1, 3, 6, and 12 months at an outpatient clinic visit or by telephone call.
STATISTICAL ANALYSIS
The primary analysis was a non-inferiority comparison of six- and 12-month DAPT for the primary MACE endpoint. We assumed that the 12-month incidence of the primary endpoint, including cardiac death, target vessel-related MI, or ischaemia-driven TLR using 12-month DAPT after the procedure, would be 7% with a similar efficacy profile of both stents13,14. A non-inferiority margin of 4% was chosen based on previous study results, clinically acceptable relevance, and the feasibility of study recruitment15. We estimated that, with a total of 1,278 patients (639 per group), the study power to reveal non-inferiority would be 80%, with a one-sided type I error rate of 0.025. We used an estimated dropout rate of 5%, which resulted in 673 patients needed for each arm.
The primary analysis was performed using intention-to-treat analysis. The analysis examined whether six-month DAPT was inferior to 12-month DAPT, with respect to the first occurrence of the primary endpoint. Kaplan-Meier survival curves for cumulative incidence of MACE at 12 months were constructed and were compared using log-rank tests. Landmark analysis was performed using a clopidogrel discontinuation landmark of six months among the patients who were event-free at six months16.
The results for analysis of categorical variables were reported as numbers and percentages, which were compared using the χ² test or Fisher’s exact test. Results for analysis of continuous variables were reported as mean±standard deviation (SD) or median (interquartile range [IQR]) values, and were compared using the Student’s t-test or the Mann-Whitney test. A p-value <0.05 was considered to indicate a statistically significant result.
Results
Between April 2011 and May 2014, each of the 1,368 patients was randomly assigned to six-month (684 patients) or 12-month (684 patients) DAPT after stent implantation. Figure 1 presents the flow diagram of the study design. One patient in the six-month group withdrew consent immediately after stent implantation and was not included in the final analysis. Seventy-three patients in the six-month DAPT group continued DAPT for more than six months; one patient in the 12-month group stopped DAPT before 12 months. The results for the baseline characteristics were not significantly different between patients crossing over to the other assigned strategy and those following the assigned strategy.
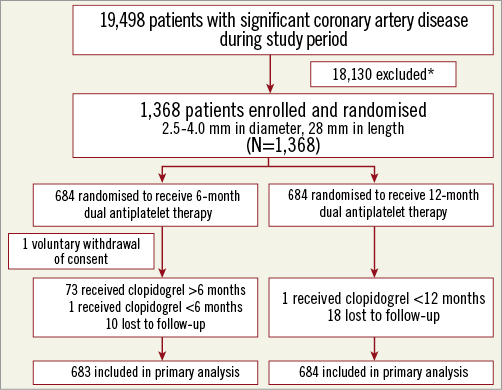
Figure 1. Study design flow chart. All patients were included in the primary “time-to-event” analysis for the duration of their follow-up, including the patients who were lost to follow-up. *Specific reasons for ineligibility were not recorded.
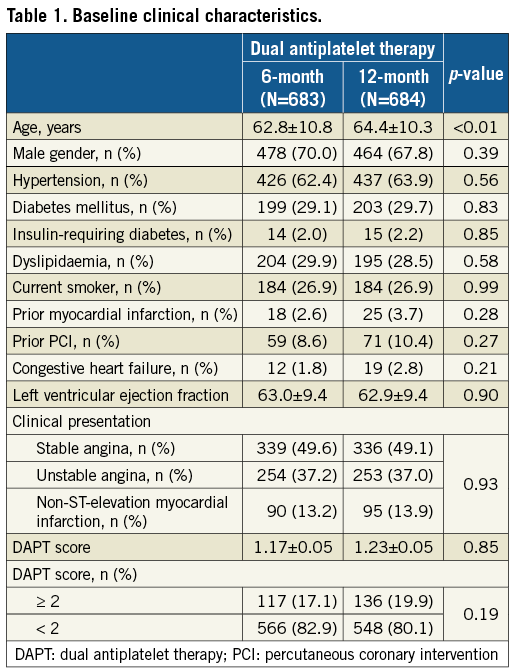
BASELINE CLINICAL CHARACTERISTICS
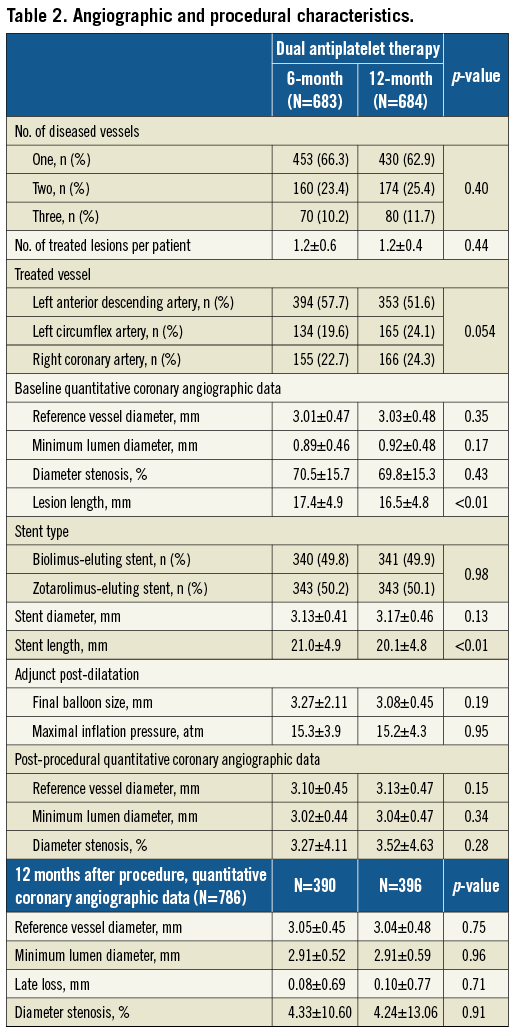
The baseline clinical characteristics were well matched in both groups, but the mean age of the patients who received six-month DAPT was less than that of those who received 12-month DAPT (62.8±10.8 versus 64.4±10.3 years, respectively) (Table 1). The angiographic and procedural characteristics were similar between the two groups, except for lesion and stent length. Mean lesion and stent lengths were longer in the six-month DAPT group compared to the 12-month DAPT group (17.4±4.9 vs. 16.5±4.8 mm, respectively, for lesion length; 21.0±4.9 versus 20.1±4.8 mm, respectively, for stent length) (Table 2). The between-group differences for the post-procedural quantitative angiography results were not statistically significant (Table 2).
CLINICAL OUTCOMES
The primary endpoint of MACE at 12 months occurred in eight (1.2%) patients from the six-month, and in four (0.6%) patients from the 12-month DAPT group (risk difference, 0.6%; 95% confidence interval [CI]: -0.4–1.6%); hazard ratio [HR] 2.02, 95% CI: 0.61-6.72; p=0.24). After adjusting for age and lesion length, the MACE rate was not significantly different (HR 2.60, 95% CI: 0.69-9.84) (Figure 2, Supplementary Table 1). For each endpoint, the total numbers for incidence of all-cause death and cardiac death were two (0.3%) and one (0.2%), respectively, for the six-month group, and three (0.4%) and two (0.3%), respectively, for the 12-month group. Target lesion-related MI occurred in only two patients (0.3%) in the six-month group. Ischaemia-driven TLR was required in six patients (0.9%) in the six-month group and two patients (0.3%) in the 12-month group (HR 3.03, 95% CI: 0.61-15.03; p=0.32). A six-month landmark analysis revealed no significant difference of MACE incidence between the six-month and 12-month groups (Figure 2, Supplementary Table 2). The per-protocol analysis outcomes were similar to the outcomes revealed by the intention-to-treat analysis (Supplementary Table 3, Supplementary Figure 1). In sensitivity analysis according to type of stent, the primary endpoint was not different between the two stents (Supplementary Table 4, Supplementary Table 5).
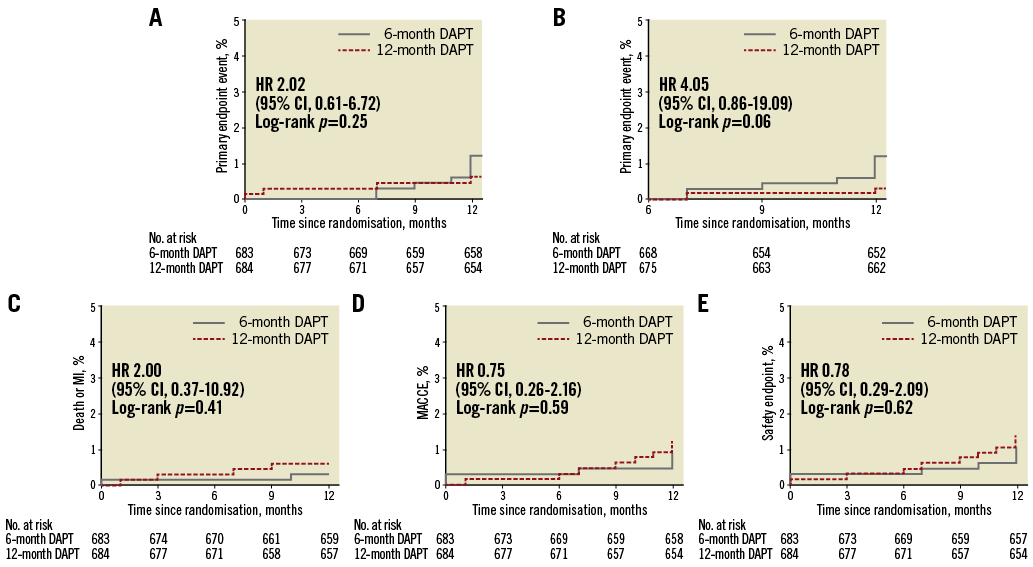
Figure 2. Kaplan-Meier curves for the primary and secondary endpoints by intention to treat. A) Primary endpoint of cardiac death, target lesion-related myocardial infarction (MI), and target lesion revascularisation. B) Six-month landmark analysis for primary endpoint. C) A composite of death or MI. D) Major adverse cardiac and cerebral events (MACCE: a composite of death, MI, stroke, or any revascularisation). E) Safety endpoint (a composite of death, myocardial infarction, stroke, stent thrombosis, or TIMI major bleeding). CI: confidence interval; HR: hazard ratio
FOLLOW-UP ASSESSMENT OF CORONARY ANGIOGRAPHY AND OCT
Approximately 60% of patients underwent follow-up coronary angiography by the end of the 12 months. The difference in late lumen loss was not statistically significant (Table 2).
OCT examination of 60 stents in 60 patients revealed an acceptable uncovered strut and malapposition rate at six months (% uncovered struts, 3.16±4.30%; % malapposed struts, 0.66±1.80%). Forty-eight (80.0%) of 60 stents had <5.9% uncovered stent struts. There were no significant differences in OCT parameters, including uncovered strut rate and malapposition rate, between the BES and ZES groups (Figure 3, Supplementary Table 6).
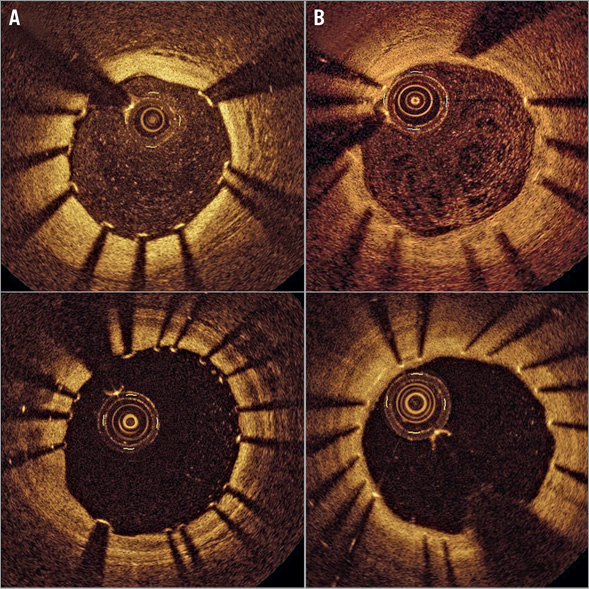
Figure 3. Representative post-intervention and six-month follow-up optical coherence tomography images. A) Post-intervention and B) six-month follow-up. At six months, favourable strut coverage was observed in biolimus-eluting stents and zotarolimus-eluting stents.
Discussion
The results of this randomised trial revealed that, after second-generation DES implantation, the use of six-month DAPT did not significantly increase MACE, including cardiac death, MI, or ischaemia-driven TLR, compared with 12-month DAPT. The OCT findings indicated acceptable stent strut coverage at six months; this result supported the safety of six-month DAPT. However, the overall event rate was 11/1,367 (0.8%), which was much lower than expected; this result should be interpreted cautiously. Based on this result, the use of a current-generation DES can be expected to result in a favourable clinical outcome when the stent is appropriately implanted in a lesion ≤28 mm and the duration of DAPT is adequate.
DAPT (including aspirin and the use of a P2Y12 receptor antagonist) is an important strategy to prevent stent thrombosis that can occur after PCI with stent implantation1. The recommendations for DAPT duration vary according to stent type3. The recommendation for duration after first-generation DES implantation was initially three to six months of DAPT, based on the drug elution period. However, a high incidence of late stent thrombosis, if DAPT was discontinued within 12 months, was consistently revealed by clinical studies in first-generation DES1. Recent studies of second-generation DES have revealed very low rates of stent-related cardiovascular events, irrespective of DAPT duration. However, most of the studies did not have sufficient sample size to assess the clinical outcomes6-8,15. A meta-analysis was performed to evaluate the optimal DAPT duration after implantation of current-generation DES. The results indicated that a shorter DAPT duration (≤6 months) was similar in safety to a longer DAPT duration (12 months)17. Updated PCI guidelines suggest that six-month DAPT can be recommended for patients with stable angina and for patients with a high bleeding risk3. However, more evidence is needed to confirm the safety of shorter duration (<6 months) DAPT after PCI using the improved profile current-generation DES.
Different types of second-generation DES have been used in previous randomised trials investigating optimal DAPT duration6-8. The everolimus-eluting stent (EES) was used in most of the studies, and in various clinical situations. However, only limited results for zotarolimus-eluting and biolimus-eluting stents and DAPT duration have been published. The RESOLUTE all-comers trial compared a ZES (Resolute™; Medtronic) and an EES (XIENCE®; Abbott Vascular, Santa Clara, CA, USA); the values for the incidence of target lesion failure at 12 months were similar (ZES, 8.2%; EES, 8.3%)14. The LEADERS study was an “all-comers” randomised trial in which the patients were randomly assigned to a BES (BioMatrix™) or a first-generation sirolimus-eluting stent (SES) (CYPHER Select®; Cordis, Cardinal Health, Milpitas, CA, USA) group. The results indicated a trend in lower MACE rates at 12 and 24 months (10.7% and 13.0%, respectively) for the BES group, compared with the SES group (12.1% and 15.4%, respectively). BES performance, in terms of strut coverage, was also better: the BES group patients had fewer lesions with at least 5% uncovered struts (3.6% versus 39.4% for the BES versus the SES group, respectively, p=0.005)13. Another study revealed similar efficacy and safety outcomes for BES compared to EES (BES, 5.2%; EES, 4.8%)18.
Similar studies that compared short (≤6 months) and long (>12 months) duration DAPT have been published. However, our study is unique because we included BES and ZES, as well as OCT examination, at six months to assess stent healing status in the same setting. Recent randomised trials did not reveal any significant differences in the values for the incidence of ischaemic events, but did find an increased bleeding risk for extended duration DAPT (12-24 months) compared with short duration DAPT (three to six months)15,19. The event rates for our treatment groups were 0.6% and 1.2% in the 12-month and six-month groups, respectively, even after ischaemia-driven TLR was included as a component of MACE. Similarly, recent randomised clinical trials have revealed lower event rates than those assumed by the investigators at the study design stage6,7. One explanation for these results is that advanced stent technology with optical stent implantation contributes to an improved vascular healing process that is associated with favourable clinical outcomes.
An extended DAPT strategy (>12 months) is still appropriate in patients with a high risk of stent thrombosis and non-stented events, even with the current second-generation DES20. The optimal DAPT duration after second-generation DES implantation remains uncertain, and stratification by the ischaemia and bleeding risks may be a more valid approach12.
We included a unique OCT substudy in our investigation. It revealed a favourable stent healing pattern (i.e., approximately 3% uncovered struts). A previous OCT registry study result indicated that a <5.9% uncovered strut rate is associated with the prevention of stent-related cardiovascular events21. Based on this result, the OCT finding in our study may provide an explanation to support the lower clinical event rate and the safety of short duration (≤6 months) DAPT to reduce the risk of stent thrombosis. The difference in late loss between six- and 12-month DAPT among the patients who agreed to follow-up coronary angiography was not statistically significant.
Limitations
There were some limitations to this study. First, the overall event rate was 0.8%, which was much lower than our expected rate of 7%. Although the difference was within the non-inferiority margin and the follow-up rate was acceptable based on study design, our study was underpowered for the possibility of showing no statistical difference according to DAPT duration due to the low MACE rate. The non-inferiority margin of 4.0% was a wide margin, considering the real events rate obtained in our study. Second, this study was open-label, and TLR was included as a primary endpoint. Third, randomisation was performed at the time of coronary angiography, and not at six months. MACE occurring in the first six months during continuation of DAPT would induce bias in the final results. Additionally, 10.7% of patients in the six-month DAPT group continued treatment for more than six months. Fourth, potent antiplatelet agents such as ticagrelor or prasugrel were not used in this study. Fifth, in this study complex lesions including left main, bifurcation, chronic total occlusion and long lesions were excluded, and the findings of the current study cannot be applied to these categories of lesion. Finally, an OCT substudy was performed in selected patients who agreed to a six-month coronary angiography and OCT examination. Extrapolation of these findings should be done with caution due to possible selection bias effects.
Conclusions
When used after second-generation DES implantation, six-month DAPT was not inferior to 12-month DAPT in terms of MACE occurrence during the 12-month follow-up period. OCT examination revealed favourable stent strut coverage at six months after stent implantation. However, the results of this study should be confirmed by large-scale studies.
| Impact on daily practice Based on the current study, six-month DAPT can be a reasonable strategy in patients with low ischaemia risk following second-generation DES implantation. OCT findings can be used to provide evidence of favourable stent healing at six months. However, further research is needed to clarify the clinical and procedural benefits to patients who receive short-term DAPT. The optimal DAPT duration should be defined to balance the ischaemia and bleeding risks after second-generation DES implantation. |
Funding
This study was supported by the Cardiovascular Research Center, Seoul, South Korea and was funded by Medtronic Vascular Inc. and Biosensors.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. List of participating centres and investigators.
Supplementary Appendix 2. Inclusion and exclusion criteria.
Supplementary Table 1. Clinical outcomes at 12 months.
Supplementary Table 2. Landmark analysis.
Supplementary Table 3. Clinical outcomes at 1 year (per-protocol analysis).
Supplementary Table 4. Clinical outcomes at 1 year in patients treated with a zotarolimus-eluting stent (ITT analysis).
Supplementary Table 5. Clinical outcomes at 1 year in patients treated with a biolimus-eluting stent (ITT analysis).
Supplementary Table 6. Optical coherence tomographic findings at 6 months after stent implantation.
Supplementary Figure 1. Kaplan-Meier curves for the primary and secondary endpoints by per-protocol analysis.
To read the full content of this article, please download the PDF

