Abstract
Background: Data on direct comparison between various drug-eluting stents with short duration dual antiplatelet therapy (DAPT) are limited, especially in patients with acute coronary syndrome (ACS).
Aims: We sought to compare biodegradable polymer biolimus-eluting stents (BP-BES) with durable polymer everolimus-eluting (DP-EES) and zotarolimus-eluting stents (DP-ZES) in patients with ACS according to different durations of DAPT.
Methods: In the SMART-DATE trial, 2,712 patients with ACS underwent randomisation for allocation of DAPT (6 months [n=1,357] or 12 months or longer [n=1,355]) and type of stent (BP-BES [n=901]), DP-EES [n=904], or DP-ZES [n=907]). The primary endpoint was a composite of cardiac death, myocardial infarction, or stent thrombosis.
Results: At 18 months, the primary endpoint was attained by 2.6% with BP-BES, 2.0% with DP-EES, and 2.1% with DP-ZES (HR 1.29, 95% CI: 0.70-2.39, p=0.42 for BP-BES vs DP-EES and HR 1.23, 95% CI: 0.67-2.26, p=0.50 for BP-BES vs DP-ZES). The treatment effect of BP-BES for the primary endpoint was consistent among patients receiving 6-month DAPT as well as those receiving 12-month or longer DAPT (BP-BES vs. DP-EES, pinteraction=0.48 and BP-BES vs DP-ZES, pinteraction=0.87). After excluding 179 patients (101 in the BP-BES group) who did not receive allocated DES, the per-protocol analysis showed similar results.
Conclusions: The risk of a composite of cardiac death, myocardial infarction, or stent thrombosis was not significantly different between patients receiving BP-BES versus DP-EES or DP-ZES across a short or prolonged duration of DAPT after ACS.
Introduction
Current major guidelines recommend dual antiplatelet therapy (DAPT) of aspirin plus a P2Y12 inhibitor for six months in patients with stable ischaemic heart disease and 12 months or longer in patients with an acute coronary syndrome (ACS) after implantation of drug-eluting stents (DES)1. However, prolonged DAPT increases the risk of bleeding that is associated with mortality2. Given the advances which have been made in stent technology in terms of alloy, strut thickness, and polymer, a case for shortening the duration of DAPT has been made. Therefore, several recent studies sought to investigate the safety of a short duration of DAPT in patients undergoing percutaneous coronary intervention (PCI) with current-generation DES3,4,5. However, data on direct comparison between various DES with a short duration of DAPT are very limited, especially in patients with ACS.
The Smart Angioplasty Research Team: safety of six-month duration of Dual Antiplatelet Therapy after Percutaneous Coronary Intervention in Patients with Acute Coronary Syndromes (SMART-DATE) trial was conducted to test the non-inferiority of six-month DAPT compared to 12-month or longer DAPT for major adverse cardiac and cerebrovascular events (MACCE) after ACS in patients undergoing PCI6. In this trial, patients with ACS underwent randomisation for allocation of antiplatelet therapy (6-month DAPT or 12-month or longer DAPT) and type of stent: stainless steel biodegradable polymer biolimus-eluting stent (BP-BES) (BioMatrix Flex™; Biosensors, Singapore); cobalt-chromium durable polymer everolimus-eluting stent (DP-EES) (XIENCE PRIME®; Abbott Vascular, Santa Clara, CA, USA); and cobalt-chromium durable polymer zotarolimus-eluting stent (DP-ZES) (Resolute Integrity®; Medtronic Vascular, Santa Rosa, CA, USA). The three types of DES used in the SMART-DATE trial have unique characteristics in terms of polymer as well as stent alloy and drugs. Therefore, this study sought to compare the outcomes of BP-BES with DP-EES and DP-ZES in patients with ACS according to different durations of DAPT. We also investigated the safety of a short duration of DAPT in each type of stent.
Methods
STUDY DESIGN AND PATIENTS
The SMART-DATE trial was a multicentre, open-label, non-inferiority, randomised trial conducted at 31 sites in the Republic of Korea to test the non-inferiority of 6-month DAPT compared with 12-month or longer DAPT following PCI with current-generation DES for ACS. The rationale and design of the SMART-DATE trial have been published previously7. The trial was designed by the steering committee and was coordinated by the Academic Clinical Research Organisation of Samsung Medical Center (Seoul, Republic of Korea). The institutional review board of each participating hospital approved the study. This trial was registered with ClinicalTrials.gov, NCT01701453. The independent data and safety monitoring board reviewed safety data from the study and provided recommendations for adverse events or serious adverse events, protocol deviation, and follow-up case reports. All patients provided written informed consent. The aim of the present analysis was to compare clinical outcomes among BP-BES, DP-EES, and DP-ZES according to the duration of DAPT. Whether the treatment effect of 6-month DAPT compared with 12-month or longer DAPT was consistent for each type of DES was also investigated.
Patients were eligible for enrolment if they had ACS that included ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina. Patients had to have at least one lesion in a native coronary vessel with reference diameter of 2.25-4.25 mm and stenosis of more than 50% by visual estimation amenable for PCI. Major exclusion criteria were a known hypersensitivity or contraindication to aspirin, clopidogrel, heparin, biolimus, everolimus, zotarolimus, or contrast media; active pathological bleeding; major bleeding within the previous three months; or major surgery within the previous two months; history of bleeding diathesis or known coagulopathy; life expectancy less than two years; an elective surgical procedure planned within less than twelve months; and active participation in another drug or device investigational study.
RANDOMISATION
Patients were randomly assigned to either the 6-month DAPT group (aspirin plus a P2Y12 inhibitor for six months and thereafter aspirin alone) or to the 12-month or longer DAPT group (aspirin plus a P2Y12 inhibitor for at least 12 months) at the time of index procedure in a 1:1 ratio. Randomisation was stratified by site of enrolment, diabetes mellitus, type of P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor), and clinical presentation (STEMI, NSTEMI, or unstable angina). To minimise bias from different stent devices, patients were also randomly assigned to one of the three DES (BP-BES, DP-EES, or DP-ZES) in a 1:1:1 ratio. The process for randomisation of type of stent was identical to that for randomisation of antiplatelet therapy. Both randomisations were carried out simultaneously via a web-based system (http://www.ecrf.kr/smartdate/login.asp) by computer-generated block method. In all lesions attempts were made to use the allocated stents for treatment, but other stents were allowed in case of device failure or in situations in which the operators decided otherwise, considering the best interests of the patient. In a minority of centres where not all three types of stents were available, especially during the early period of the trial, available study stents had to be implanted instead of the allocated stents at the discretion of the operators.
STUDY ENDPOINTS
The primary endpoint was a composite of cardiac death, MI, or stent thrombosis at 18 months after the index procedure. Secondary endpoints were the individual components of the primary endpoint, all-cause death, target lesion revascularisation (TLR) or target lesion failure (TLF). TLF was defined as a composite of cardiac death, MI, or TLR. An independent clinical events adjudication committee, whose members were masked to the study group assignments, assessed all clinical endpoints.
All deaths were considered cardiac unless a definite non-cardiac cause could be established. MI was defined as elevated cardiac enzymes (cardiac troponin or myocardial band fraction of creatine kinase) above the upper reference limit with ischaemic symptoms or electrocardiography findings indicative of ischaemia that was not related to the index procedure. Stent thrombosis was defined as definite or probable stent thrombosis according to the Academic Research Consortium classification8.
PROCEDURES AND STATISTICAL ANALYSIS
Procedures and statistical analysis are described in detail in Supplementary Appendix 1.
Results
BASELINE CHARACTERISTICS
Between September 2012 and December 2015, a total of 2,712 patients were enrolled in the SMART-DATE trial. Of these, 901 patients were randomly assigned to receive BP-BES, 904 were randomly assigned to receive DP-EES, and 907 were randomly assigned to receive DP-ZES. However, a substantial number of patients did not receive the allocated stents. Especially in the BP-BES group, 101 patients did not receive BP-BES: 5 underwent ballooning or thrombus aspiration only, and 96 received other DES due to unavailability of the allocated stent of the proper size or length (n=49), failure of delivery (n=3), operators’ discretion (n=10) or unknown cause (n=34). A total of 34 patients in the DP-EES and 44 patients in the DP-ZES group did not receive the allocated stents (Figure 1). Patients in the three DES groups were balanced for all baseline demographic and clinical characteristics (Supplementary Table 1). The mean age was 62 years and 75.4% of the overall population were male. Twenty-seven point five percent (27.5%) of the study population suffered from diabetes mellitus and 37.7% of overall patients presented with STEMI. Angiographic and procedural data were similar in the three groups (Supplementary Table 2). Multivessel disease was identified in 45.1% of all study patients, and the left main or left anterior descending artery lesion was treated in 60.5%. Although the mean stent length per lesion was significantly different among the three groups, the difference was not substantial (23.9±6.5 mm with BP-BES vs 25.1±7.6 mm with DP-EES vs 25.0±7.1 mm with DP-ZES; p<0.01).
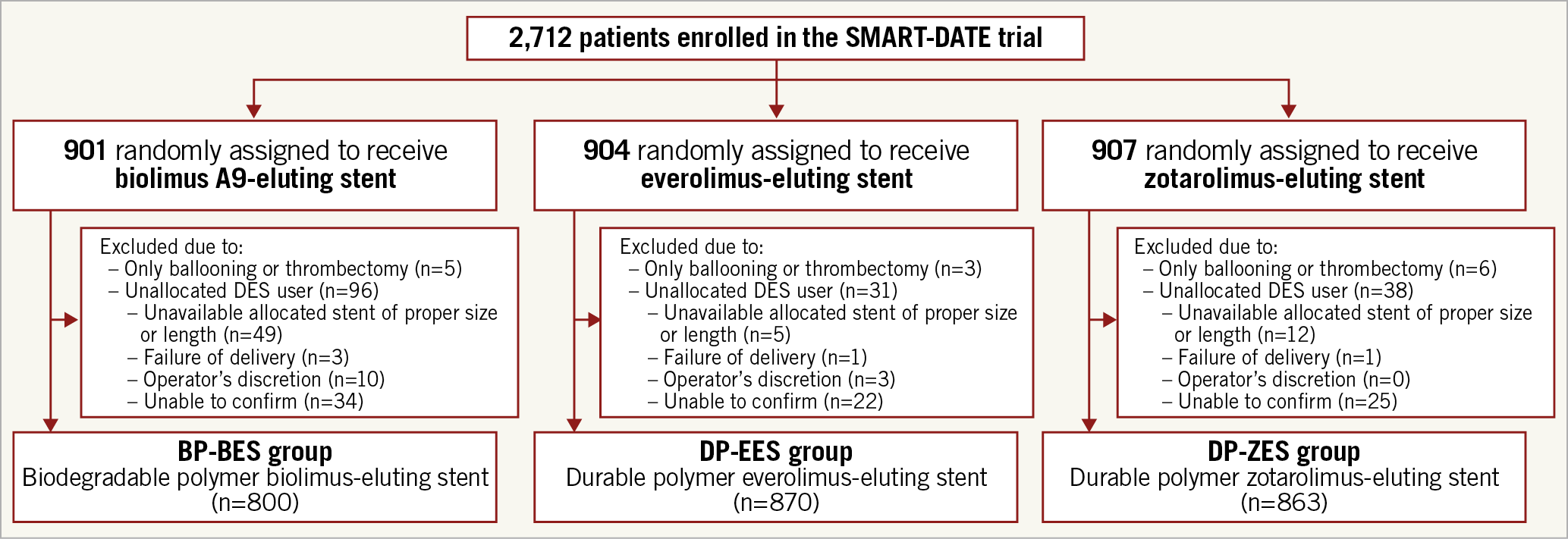
Figure 1. Schematic illustration of the study cohort selection.
ANTIPLATELET THERAPY
Of 2,712 patients, 1,357 were assigned to the 6-month DAPT group and 1,355 were assigned to the 12-month or longer DAPT group. Clopidogrel was used as a P2Y12 inhibitor for DAPT in 2,191 patients (80.8%). The adherence rate of assigned antiplatelet therapy was not significantly different according to the type of stent (87.4% for the BP-BES group vs 85.9% for the DP-EES group vs 87.2% for the DP-ZES group; p=0.54). Neither the duration of DAPT (p=0.50) nor the type of P2Y12 inhibitor (p=0.30) differed significantly among the three DES groups.
COMPARISON AMONG THE THREE TYPES OF STENT
Follow-up for the primary endpoint at 18 months was completed in 97.4% of all patients (97.1% for the BP-BES group vs 96.9% for the DP-EES group vs 98.0% for the DP-ZES group; p=0.29). At 18 months after the index procedure, the primary endpoint occurred in 23 patients in the BP-BES group, 18 in the DP-EES group, and 19 in the DP-ZES group. Cumulative rates of the primary endpoint at 18 months were 2.6% for the BP-BES group, 2.0% for the DP-EES group, and 2.1% for the DP-ZES group (hazard ratio [HR] for BP-BES vs DP-EES 1.29, 95% confidence interval [CI]: 0.70-2.39; p=0.42 and HR for BP-BES vs DP-ZES 1.23, 95% CI: 0.67-2.26; p=0.50) (Table 1, Figure 2). There were no significant differences in the individual components of the primary endpoint, TLR and TLF at 18 months. Cumulative rates of all-cause death were numerically higher in the BP-BES group (3.7%) than in the DP-EES (2.1%) and the DP-ZES groups (2.4%), but statistical significance was not found (HR for BP-BES vs DP-EES 1.75, 95% CI: 0.99-3.08; p=0.052, and HR for BP-BES vs DP-ZES 1.53, 95% CI: 0.89-2.62; p=0.13) (Table 1). For per-protocol analysis, 179 patients who did not receive the assigned stents were excluded. A total of 800 patients in the BP-BES group, 870 in the DP-EES group, and 863 in the DP-ZES group were included (Figure 1). The results from per-protocol analysis were similar to those from intention-to-treat analysis. The cumulative rates of the primary endpoint at 18 months were 2.6% for the BP-BES group, 2.0% for the DP-EES group, and 2.0% for the DP-ZES group (HR for BP-BES vs DP-EES 1.35, 95% CI: 0.71-2.56; p=0.36, and HR for BP-BES vs DP-ZES 1.35, 95% CI: 0.71-2.56; p=0.36). There were no significant differences in the individual components of the primary endpoint, all-cause death, TLR, and TLF, among the three types of stent at 18 months (Supplementary Table 3). The treatment effect of BP-BES for the primary endpoint was consistent among patients receiving 6-month DAPT as well as those receiving 12-month or longer DAPT compared with the other two stents (Supplementary Table 4, Supplementary Figure 1). Landmark analysis at six months showed that the risk of the primary endpoint between 6 and 18 months was not significantly different among the three DES groups (Supplementary Appendix 2, Supplementary Figure 2).
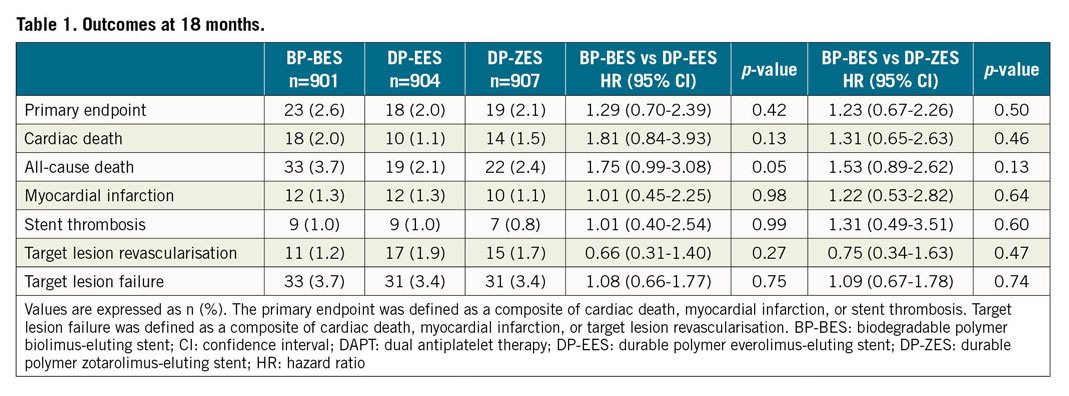
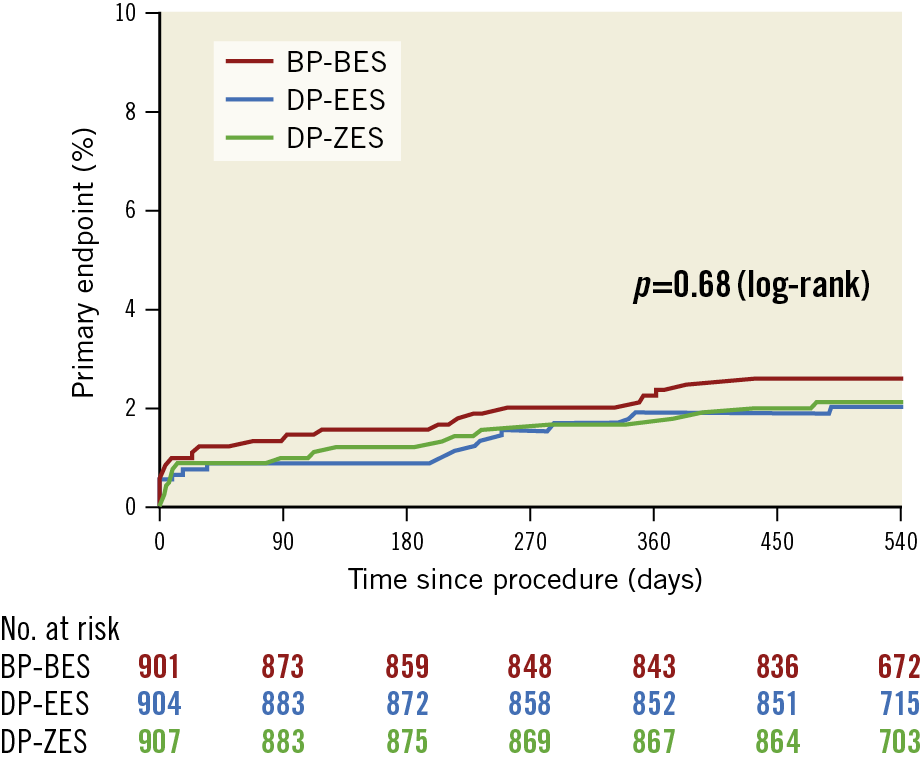
Figure 2. Time-to-event Kaplan-Meier curves for the primary endpoint. BP-BES: biodegradable polymer biolimus-eluting stent; DP-EES: durable polymer everolimus-eluting stent; DP-ZES: durable polymer zotarolimus-eluting stent
TREATMENT EFFECT OF SHORT DURATION DAPT IN EACH STENT
The treatment effect of the duration of DAPT for the primary endpoint (pinteraction=0.49) and TLF (pinteraction=0.75) was consistent in each stent type. The risk of the primary endpoint was not significantly different between 6-month DAPT and 12-month or longer DAPT in each stent type (2.5% 6-month DAPT vs 2.6% 12-month DAPT, HR 0.95: 0.42-2.15; p=0.90 for BP-BES group, 2.8% vs 1.1%; HR 2.54: 0.91-7.12; p=0.08 for DP-EES group, 2.4% vs 1.8%; HR 1.37: 0.55-3.42; p=0.49 for DP-ZES group, respectively) and the rate of TLF was also similar (3.4% 6-month DAPT vs 3.9% 12-month DAPT; HR 0.86: 0.44-1.71; p=0.67 for BP-BES group, 4.6% vs 2.2%; HR 2.06: 0.97-4.38; p=0.06 for DP-EES group, 2.9% vs 4.0%; HR 0.72: 0.35-1.47; p=0.37 for DP-ZES group, respectively) (Supplementary Table 5). A per-protocol analysis in terms of antiplatelet assignment showed a consistent treatment effect of BP-BES for the primary endpoint (HR for BP-BES vs DP-EES 2.25, 95% CI: 0.69-7.31; p=0.18, and HR for BP-BES vs DP-ZES 1.32, 95% CI: 0.49-3.55; p=0.36, respectively).
Discussion
In the present study, we compared BP-BES with DP-EES and DP-ZES and evaluated the safety of 6-month DAPT in patients with ACS undergoing PCI. Our main finding was that the composite of cardiac death, MI, or stent thrombosis at 18 months did not differ in ACS patients receiving BP-BES compared with those receiving DP-EES or DP-ZES. The treatment effect of BP-BES, compared with DP-EES and DP-ZES, was consistent among patients receiving 6-month DAPT as well as those receiving 12-month or longer DAPT for the composite of cardiac death, MI, or stent thrombosis. In all three types of DES, 6-month DAPT was not associated with increased risk of device-specific outcomes compared with 12-month or longer DAPT.
Several previous studies demonstrated that DES with a short duration of DAPT was superior compared with bare metal stents. The LEADERS FREE trial first revealed that a polymer-free biolimus-coated stent followed by one-month DAPT reduced major adverse cardiac events compared with bare metal stents in patients at high bleeding risk3. In the SENIOR trial, the SYNERGY™ platinum-chromium EES (Boston Scientific, Marlborough, MA, USA) with biodegradable polymer and a short duration of DAPT were superior to bare metal stents and a similar duration of DAPT for all-cause death, MI, stroke, and ischaemia-driven TLR among elderly patients9. However, to date, there has been only one trial which compared different types of DES with a short duration of DAPT5. In the SMART-DATE trial, patients with ACS were exclusively enrolled and were randomly assigned to 6-month DAPT or 12-month or longer DAPT. Furthermore, type of stent was also randomly allocated. Therefore, we had a unique opportunity to compare BP-BES with DP-EES and DP-ZES across short and prolonged DAPT regimens in patients with ACS. Although the current-generation DES are excellent and comparable to each other, it would be valuable to compare outcomes of various DES with a short duration of DAPT and investigate the safety of short-duration DAPT in each stent, especially among patients at high risk, such as those with ACS. We believe that our study addresses important issues and can add new knowledge on the optimal duration of DAPT in patients with ACS undergoing PCI in contemporary practice.
The first-generation DES dramatically reduced restenosis and TLR compared with bare metal stents; however, it was reported to be associated with increased risk of very late stent thrombosis10. Of several plausible explanations, inflammation induced by polymer might play an important role for stent thrombosis related to first-generation DES11. As expected, BP-DES have demonstrated a reduced risk of very late stent thrombosis compared with the first-generation DES12. Moreover, in randomised clinical trials and observational studies comparing BP-BES with the second-generation DES13,14, no significant differences were observed. However, a couple of network meta-analyses reported that BP-BES were associated with a higher risk of definite or probable stent thrombosis, MI, and mortality compared with the second-generation DES15,16. Although the BioMatrix Flex BP-BES is no longer widely used in contemporary practice, it is the prototype of BP-DES and controversy remains regarding the safety of BP-DES. Taken together, to compare BP-BES with DP-DES with documented safety according to different DAPT durations is of great importance. In the present study, the risk of cardiac death, MI, or stent thrombosis was not significantly different among the three types of stent. However, cumulative rates of cardiac or all-cause death were numerically higher in the BP-BES group than in the DP-EES and DP-ZES groups. The HR for all-cause death in patients receiving BP-BES versus DP-EES in the present study was similar to that in a previous network meta-analysis16; the only difference was the width of the 95% CI. The present study might have been underpowered to detect difference in clinical outcomes among the three stents. Therefore, our results should be interpreted cautiously.
Limitations
This study has several limitations. First, a substantial number of patients in the BP-BES group did not receive the allocated stents. The possibility of biases towards similar outcomes of BP-BES compared with DP-EES and DP-ZES originating from implantation of substitutes (other DES) for BP-BES in a high-risk population or high-risk lesion subsets cannot be excluded. Weakness in the mechanical properties of BP-BES such as thick struts and a stainless steel platform might have made operators reluctant to implant the assigned BP-BES in a high-risk population or high-risk lesion subsets, especially when difficulty in delivery of the stents was expected. However, intention-to-treat and per-protocol analyses showed similar results, suggesting that any potential biases caused by crossover are probaby small. Second, this study was an open-label trial. Although all clinical endpoints were assessed by members of the independent clinical events adjudication committee, operators were not blinded to the type of stent. However, the primary endpoint of this study was a composite of cardiac death, MI, or stent thrombosis, and their occurrence was not affected by investigators and patients, unlike repeat revascularisation or hospitalisation due to unstable angina. Third, all consecutive patients could not be considered for study enrolment, which might have resulted in selection bias for enrolment of patients with relatively low risk. Moreover, the present analysis might have been underpowered due to inadequate sample size and low event rates. However, contemporary large randomised trials in patients with ACS reported similar event rates compared with the SMART-DATE trial17,18. Fourth, follow-up duration was 18 months in the present study. Because it takes nine months for polymer to be completely degraded after implantation of BP-BES19, long-term follow-up is warranted. We plan to follow up patients up to three years after the index procedure. Fifth, a substantial number of patients in the 6-month DAPT group received a P2Y12 inhibitor after six months. However, intention-to-treat and per-protocol analyses for antiplatelet therapy showed similar results. Sixth, although prasugrel or ticagrelor is recommended as the first-line therapy in patients with ACS undergoing PCI1, clopidogrel was used predominantly because prasugrel and ticagrelor became available in the Republic of Korea during the course of the study (in December 2014). Although the benefit of prolonged DAPT might be mitigated by predominant use of clopidogrel instead of prasugrel or ticagrelor, clopidogrel is still prescribed in a substantial proportion of patients with ACS4,20. Finally, we did not consider statistical correction for multiple testing in the present study since randomisation for antiplatelet therapy and randomisation for type of stent were independent. Moreover, considering that there was no significant difference among the three types of DES in the present analysis with an alpha of 0.05, adjusting for multiple comparison by using, e.g., the Bonferroni correction with an alpha of 0.025 would result in a similar conclusion.
Conclusions
The risk of a composite of cardiac death, MI, or stent thrombosis was not significantly different among patients receiving BP-BES versus DP-EES or DP-ZES across a short or prolonged duration of DAPT after ACS.
|
Impact on daily practice There remains controversy regarding the safety of BP-BES, and data are limited on the optimal duration of DAPT after implantation of BP-BES in patients with ACS who have a higher risk of recurrent ischaemic events than those with stable coronary artery disease. According to this study, the risk of a composite of cardiac death, myocardial infarction, or stent thrombosis did not differ in ACS patients receiving BP-BES compared with those receiving DP-EES or DP-ZES. The treatment effect of BP-BES, compared with DP-EES and DP-ZES, was consistent among patients receiving 6-month DAPT as well as those receiving 12-month or longer DAPT. |
Acknowledgements
We wish to thank Drs TK Park, JM Lee, JH Yang, YH Park, JH Cho, JH Bae, DI Kim and WS Lee for data collection for the SMART-DATE trial.
Funding
Grant support: Abbott Vascular Korea, Medtronic Vascular Korea, Biosensors Inc., and Dong-A ST.
Conflict of interest statement
H.C. Gwon has received research grants from Abbott Vascular, Boston Scientific, and Medtronic, and speaker’s fees from Abbott Vascular, Boston Scientific, and Medtronic. J.Y. Hahn has received grants from Abbott Vascular, Biotronik, Boston Scientific, Daiichi Sankyo, and Medtronic, and speaker’s fees from AstraZeneca, Daiichi Sankyo, and Sanofi-Aventis. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

